Figure 5.
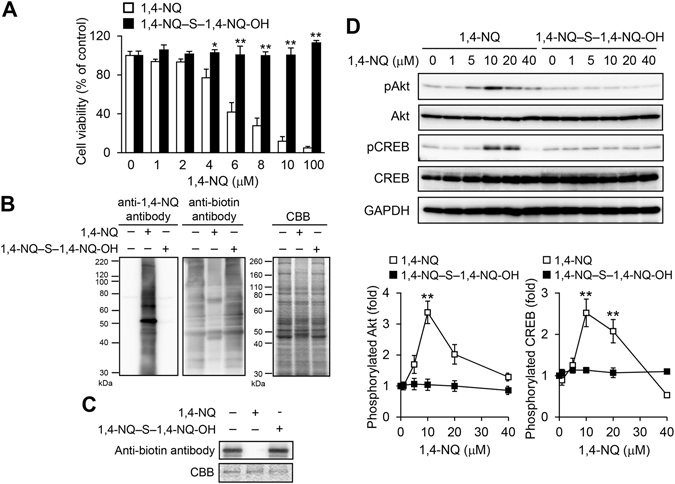
Cytotoxicity, covalent protein modification and Akt–CREB signaling activation caused by exposure of primary mouse hepatocytes to 1,4-NQ-sulfur adducts. (A) Primary mouse hepatocytes were exposed to 1,4-NQ or 1,4-NQ–S–1,4-NQ-OH for 24 h. Cell viability was determined with the MTT assay. Each value is the mean ± standard error for three independent experiments. (B) Cells were exposed to 1,4-NQ–S–1,4-NQ-OH (20 µM) or 1,4-NQ (40 µM) for 1 h. Covalent modification of cellular proteins with 1,4-NQ, as detected by western blotting (left), BPM assay results (middle) and protein bands on SDS-PAGE, as stained by Coomassie Brilliant Blue (right). Representative blots are shown from three independent experiments. (C) Recombinant GST-tagged human PTEN (1 μg) was incubated with 1,4-NQ–S–1,4-NQ-OH (5 µM) or 1,4-NQ (10 µM) at 25 °C for 1 h and then further incubated with BPM (15 μM) at 37 °C for 30 min. The reaction mixture was then analyzed by immunoblotting with the anti-biotin antibody and Coomassie Brilliant Blue was used to visualize bands after SDS-PAGE. Representative blots are shown from three independent experiments. (D) Cells were exposed to different concentrations of 1,4-NQ-sulfide for 30 min. Akt and CREB phosphorylation was determined by western blotting (upper). Representative blots are shown from three independent experiments. Band intensities were normalized to those for total Akt and CREB, respectively (lower). Intensities are expressed as fold induced, relative to results with 0 µM 1,4-NQ exposure. Each value is the mean ± the standard error for three independent experiments. *P < 0.05 and *P < 0.01, compared with the 0 µM 1,4-NQ exposure.
