Abstract
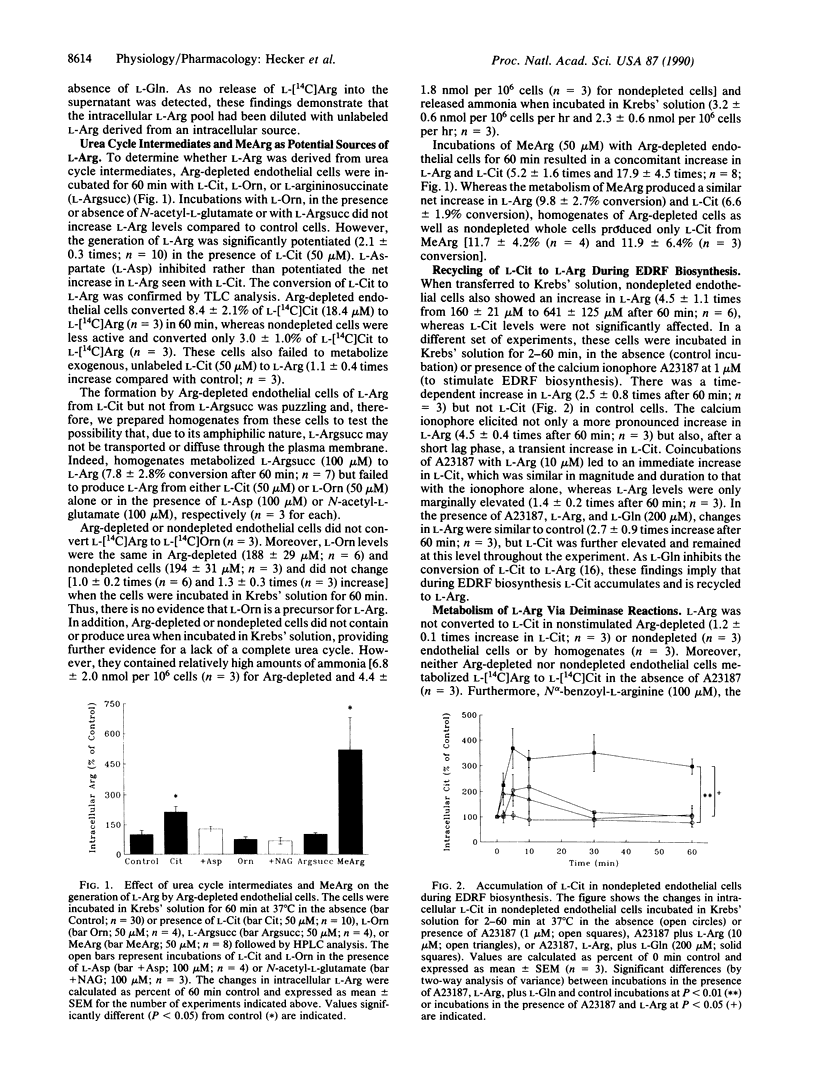
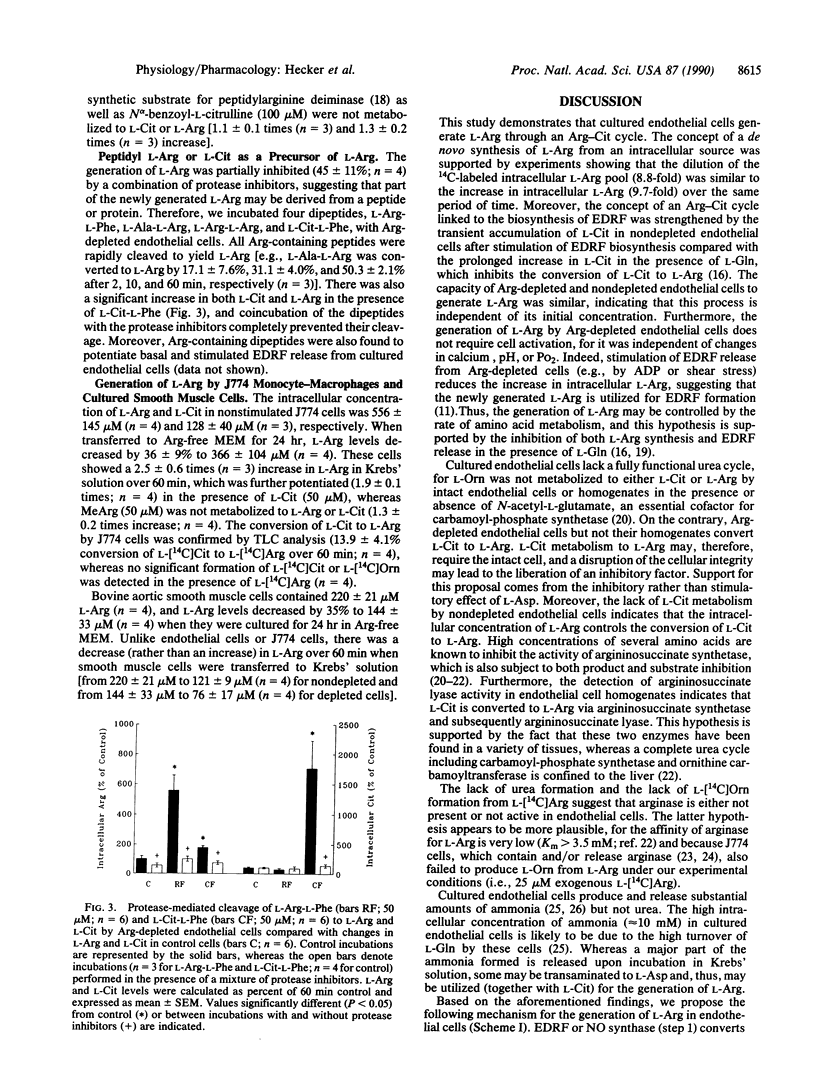
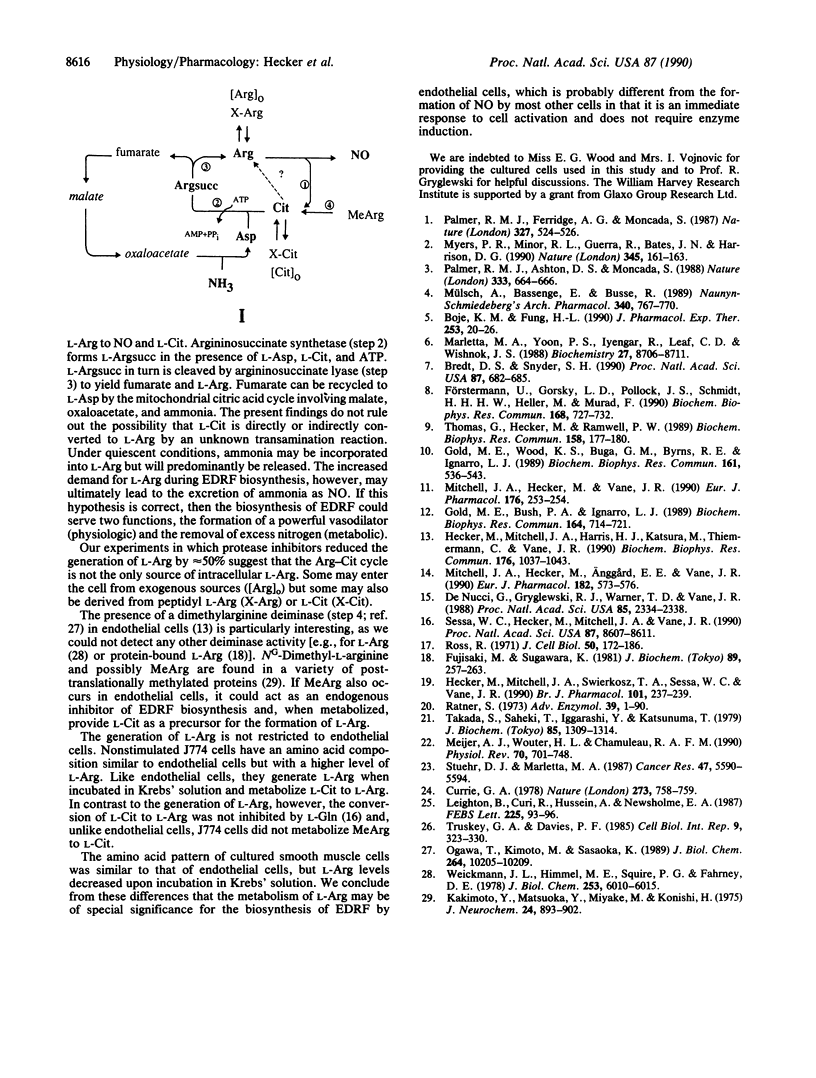
We have investigated the mechanism by which cultured endothelial cells generate L-arginine (L-Arg), the substrate for the biosynthesis of endothelium-derived relaxing factor. When Arg-depleted endothelial cells were incubated in Krebs' solution for 60 min, L-Arg levels were significantly (9.7-fold) elevated. The generation of L-Arg coincided with a substantial decrease (90%) in intracellular L-glutamine (L-Gln), whereas all other amino acids were virtually unaffected. Changes in calcium, pH, or oxygen tension had no effect on L-Arg generation, which was, however, prevented when the cells were incubated in culture medium containing L-Gln. L-Arg generated by endothelial cells labeled with L-[14C]Arg was derived from an unlabeled intracellular source, for the specific activity of the intracellular L-Arg pool decreased substantially (8.8-fold) over 60 min. Arg-depleted endothelial cells did not form urea or metabolize L-ornithine but converted L-citrulline (L-Cit) to L-Arg possibly via formation of L-argininosuccinic acid. Nondepleted cells stimulated with the calcium ionophore A23187 showed only a transient accumulation of L-Cit, indicating that L-Cit is recycled to L-Arg during the biosynthesis of endothelium-derived relaxing factor. The generation of L-Arg by Arg-depleted endothelial cells was partially (45%) blocked by protease inhibitors, and various Arg-containing dipeptides were rapidly cleaved to yield L-Arg. Thus, cultured endothelial cells recycle L-Cit to L-Arg and possibly liberate peptidyl L-Arg. The Arg-Cit cycle appears to be the equivalent in the endothelial cell to the formation of urea by the liver. The biosynthesis of endothelium-derived relaxing factor may, therefore, not only produce a powerful vasodilator but also relieve the endothelial cell of excess nitrogen.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boje K. M., Fung H. L. Endothelial nitric oxide generating enzyme(s) in the bovine aorta: subcellular location and metabolic characterization. J Pharmacol Exp Ther. 1990 Apr;253(1):20–26. [PubMed] [Google Scholar]
- Bredt D. S., Snyder S. H. Isolation of nitric oxide synthetase, a calmodulin-requiring enzyme. Proc Natl Acad Sci U S A. 1990 Jan;87(2):682–685. doi: 10.1073/pnas.87.2.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie G. A. Activated macrophages kill tumour cells by releasing arginase. Nature. 1978 Jun 29;273(5665):758–759. doi: 10.1038/273758a0. [DOI] [PubMed] [Google Scholar]
- Fujisaki M., Sugawara K. Properties of peptidylarginine deiminase from the epidermis of newborn rats. J Biochem. 1981 Jan;89(1):257–263. doi: 10.1093/oxfordjournals.jbchem.a133189. [DOI] [PubMed] [Google Scholar]
- Förstermann U., Gorsky L. D., Pollock J. S., Schmidt H. H., Heller M., Murad F. Regional distribution of EDRF/NO-synthesizing enzyme(s) in rat brain. Biochem Biophys Res Commun. 1990 Apr 30;168(2):727–732. doi: 10.1016/0006-291x(90)92382-a. [DOI] [PubMed] [Google Scholar]
- Gold M. E., Bush P. A., Ignarro L. J. Depletion of arterial L-arginine causes reversible tolerance to endothelium-dependent relaxation. Biochem Biophys Res Commun. 1989 Oct 31;164(2):714–721. doi: 10.1016/0006-291x(89)91518-0. [DOI] [PubMed] [Google Scholar]
- Gold M. E., Wood K. S., Buga G. M., Byrns R. E., Ignarro L. J. L-arginine causes whereas L-argininosuccinic acid inhibits endothelium-dependent vascular smooth muscle relaxation. Biochem Biophys Res Commun. 1989 Jun 15;161(2):536–543. doi: 10.1016/0006-291x(89)92632-6. [DOI] [PubMed] [Google Scholar]
- Hecker M., Mitchell J. A., Harris H. J., Katsura M., Thiemermann C., Vane J. R. Endothelial cells metabolize NG-monomethyl-L-arginine to L-citrulline and subsequently to L-arginine. Biochem Biophys Res Commun. 1990 Mar 30;167(3):1037–1043. doi: 10.1016/0006-291x(90)90627-y. [DOI] [PubMed] [Google Scholar]
- Hecker M., Mitchell J. A., Swierkosz T. A., Sessa W. C., Vane J. R. Inhibition by L-glutamine of the release of endothelium-derived relaxing factor from cultured endothelial cells. Br J Pharmacol. 1990 Oct;101(2):237–239. doi: 10.1111/j.1476-5381.1990.tb12693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakimoto Y., Matsuoka Y., Miyake M., Konishi H. Methylated amino acid residues of proteins of brain and other organs. J Neurochem. 1975 May;24(5):893–902. doi: 10.1111/j.1471-4159.1975.tb03653.x. [DOI] [PubMed] [Google Scholar]
- Leighton B., Curi R., Hussein A., Newsholme E. A. Maximum activities of some key enzymes of glycolysis, glutaminolysis, Krebs cycle and fatty acid utilization in bovine pulmonary endothelial cells. FEBS Lett. 1987 Dec 10;225(1-2):93–96. doi: 10.1016/0014-5793(87)81137-7. [DOI] [PubMed] [Google Scholar]
- Marletta M. A., Yoon P. S., Iyengar R., Leaf C. D., Wishnok J. S. Macrophage oxidation of L-arginine to nitrite and nitrate: nitric oxide is an intermediate. Biochemistry. 1988 Nov 29;27(24):8706–8711. doi: 10.1021/bi00424a003. [DOI] [PubMed] [Google Scholar]
- Meijer A. J., Lamers W. H., Chamuleau R. A. Nitrogen metabolism and ornithine cycle function. Physiol Rev. 1990 Jul;70(3):701–748. doi: 10.1152/physrev.1990.70.3.701. [DOI] [PubMed] [Google Scholar]
- Mitchell J. A., Hecker M., Anggård E. E., Vane J. R. Cultured endothelial cells maintain their L-arginine level despite the continuous release of EDRF. Eur J Pharmacol. 1990 Jul 17;182(3):573–576. doi: 10.1016/0014-2999(90)90058-e. [DOI] [PubMed] [Google Scholar]
- Mitchell J. A., Hecker M., Vane J. R. The generation of L-arginine in endothelial cells is linked to the release of endothelium-derived relaxing factor. Eur J Pharmacol. 1990 Feb 6;176(2):253–254. doi: 10.1016/0014-2999(90)90541-d. [DOI] [PubMed] [Google Scholar]
- Myers P. R., Minor R. L., Jr, Guerra R., Jr, Bates J. N., Harrison D. G. Vasorelaxant properties of the endothelium-derived relaxing factor more closely resemble S-nitrosocysteine than nitric oxide. Nature. 1990 May 10;345(6271):161–163. doi: 10.1038/345161a0. [DOI] [PubMed] [Google Scholar]
- Mülsch A., Bassenge E., Busse R. Nitric oxide synthesis in endothelial cytosol: evidence for a calcium-dependent and a calcium-independent mechanism. Naunyn Schmiedebergs Arch Pharmacol. 1989 Dec;340(6 Pt 2):767–770. doi: 10.1007/BF00169688. [DOI] [PubMed] [Google Scholar]
- Ogawa T., Kimoto M., Sasaoka K. Purification and properties of a new enzyme, NG,NG-dimethylarginine dimethylaminohydrolase, from rat kidney. J Biol Chem. 1989 Jun 15;264(17):10205–10209. [PubMed] [Google Scholar]
- Palmer R. M., Ashton D. S., Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988 Jun 16;333(6174):664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Ratner S. Enzymes of arginine and urea synthesis. Adv Enzymol Relat Areas Mol Biol. 1973;39:1–90. doi: 10.1002/9780470122846.ch1. [DOI] [PubMed] [Google Scholar]
- Ross R. The smooth muscle cell. II. Growth of smooth muscle in culture and formation of elastic fibers. J Cell Biol. 1971 Jul;50(1):172–186. doi: 10.1083/jcb.50.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sessa W. C., Hecker M., Mitchell J. A., Vane J. R. The metabolism of L-arginine and its significance for the biosynthesis of endothelium-derived relaxing factor: L-glutamine inhibits the generation of L-arginine by cultured endothelial cells. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8607–8611. doi: 10.1073/pnas.87.21.8607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuehr D. J., Marletta M. A. Synthesis of nitrite and nitrate in murine macrophage cell lines. Cancer Res. 1987 Nov 1;47(21):5590–5594. [PubMed] [Google Scholar]
- Takada S., Saheki T., Igarashi Y., Katsunuma T. Studies on rat liver argininosuccinate synthetase. Inhibition by various amino acids. J Biochem. 1979 May;85(5):1309–1314. [PubMed] [Google Scholar]
- Thomas G., Hecker M., Ramwell P. W. Vascular activity of polycations and basic amino acids: L-arginine does not specifically elicit endothelium-dependent relaxation. Biochem Biophys Res Commun. 1989 Jan 16;158(1):177–180. doi: 10.1016/s0006-291x(89)80194-9. [DOI] [PubMed] [Google Scholar]
- Truskey G. A., Davies P. F. Effects of ammonium ion derived from bovine endothelial cells upon low density lipoprotein degradation in cultured vascular smooth muscle cells. Cell Biol Int Rep. 1985 Apr;9(4):323–330. doi: 10.1016/0309-1651(85)90027-x. [DOI] [PubMed] [Google Scholar]
- Weickmann J. L., Himmel M. E., Squire P. G., Fahrney D. E. Arginine deiminase from Mycoplasma arthritidis. Properties of the enzyme from log phase cultures. J Biol Chem. 1978 Sep 10;253(17):6010–6015. [PubMed] [Google Scholar]
- de Nucci G., Gryglewski R. J., Warner T. D., Vane J. R. Receptor-mediated release of endothelium-derived relaxing factor and prostacyclin from bovine aortic endothelial cells is coupled. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2334–2338. doi: 10.1073/pnas.85.7.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]


