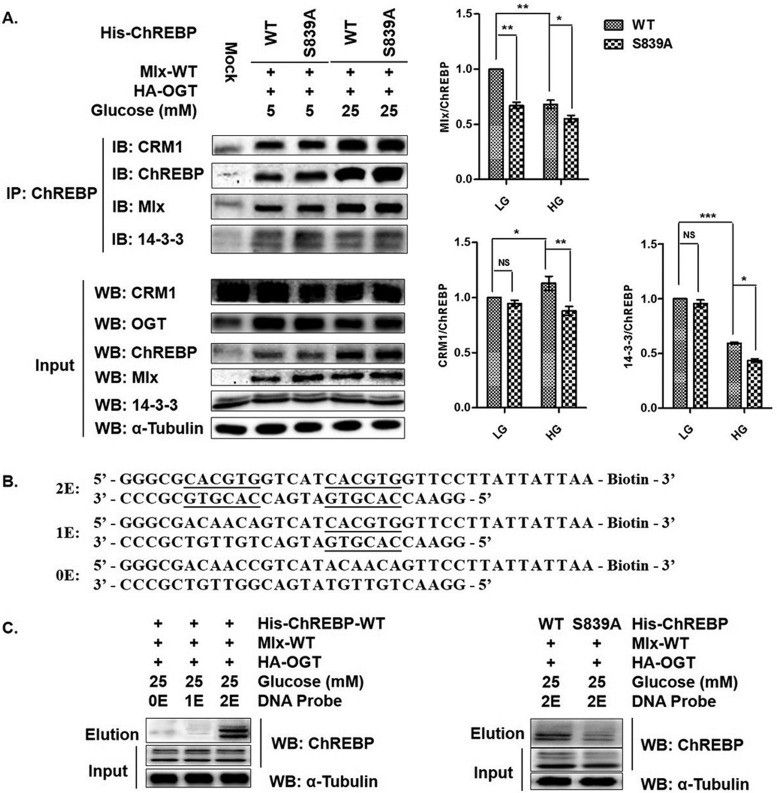
Fig. 6.
O-GlcNAcylation at Ser839 of ChREBP is important for its function. A, Either WT ChREBP or S839A ChREBP mutant was coexpressed in HEK293T with OGT and Mlx, and incubated under the indicated conditions for 12 h with TMG treatment. Coimmunoprecipitation was performed using anti-ChREBP antibody and the eluates were assessed for ChREBP and its interactors, Mlx, CRM1, and 14-3-3 by WB. Cell lysates were also immunoblotted as references. Left lane shows a representative WB result of 6 independent experiments. Right lane shows WB result in statistical result. Error bars denote the mean ± S.E., n = 6. * p < 0.05; ** p < 0.01; *** p < 0.001; NS: no significance. B, Designed DNA-binding probes for ChREBP, whose E-boxes of the glucose-responsive binding element—ChoRE—were underlined. C, Cells were transfected like A and incubated under the HG condition. Cell lysates were pulled down using biotinylated DNA probes and streptavidin-beads. Eluates and cell lysates were assessed for ChREBP by WB. α-Tubulin from cell lysates was immunoblotted as a reference. Left lane shows only 2E DNA probe could pull down ChREBP, right lane shows DNA-binding differences between WT ChREBP and S839A mutant. All data are representative of 3 independent experiments.