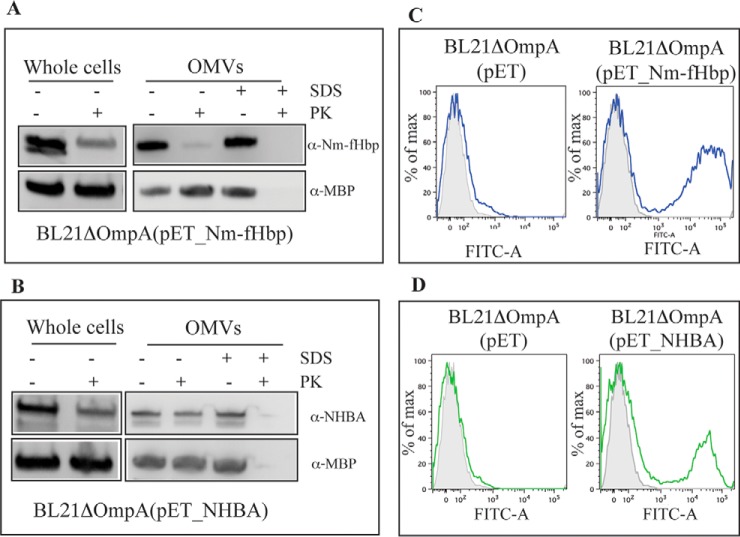
Fig. 3.
Assessment of Nm-fHbp and NHBA localization by proteinase K surface shaving and FACS analysis. A, Bacterial cells and purified OMVs from BL21ΔompA(pET_Nm-fHbp) strain were incubated at 37 °C for 2 h with and without Proteinase K (PK) in the presence or absence of 1% SDS. Integrity of Nm-fHbp under the different experimental conditions was analyzed by Western blotting using Nm-fHbp specific antibodies. The integrity of the periplasmic protein MBP was also analyzed as control. B, Bacterial cells and purified OMVs from BL21ΔompA(pET_NHBA) strain were treated as in A and the integrity of NHBA under the different experimental conditions was analyzed by Western blotting using NHBA-specific antibodies. C, Bacterial cells from BL21ΔompA and BL21ΔompA (pET_Nm-fHbp) strains were incubated first with anti-Nm-fHbp specific antibodies and subsequently with FITC-labeled anti-mouse secondary antibodies. Fluorescence was measured by Fluorescence-activated Cell Sorting (FACS). Gray areas represent the background fluorescence signals obtained incubating the cells with the secondary antibody only. D, Bacterial cells from BL21ΔompA and BL21ΔompA(pET_NHBA) strains were incubated first with anti-NHBA specific antibodies and subsequently with alexa fluor® 488-labeled anti-mouse secondary antibodies. Fluorescence was measured by FACS. Gray areas represent the background fluorescence signals obtained incubating the cells with the secondary antibody only.