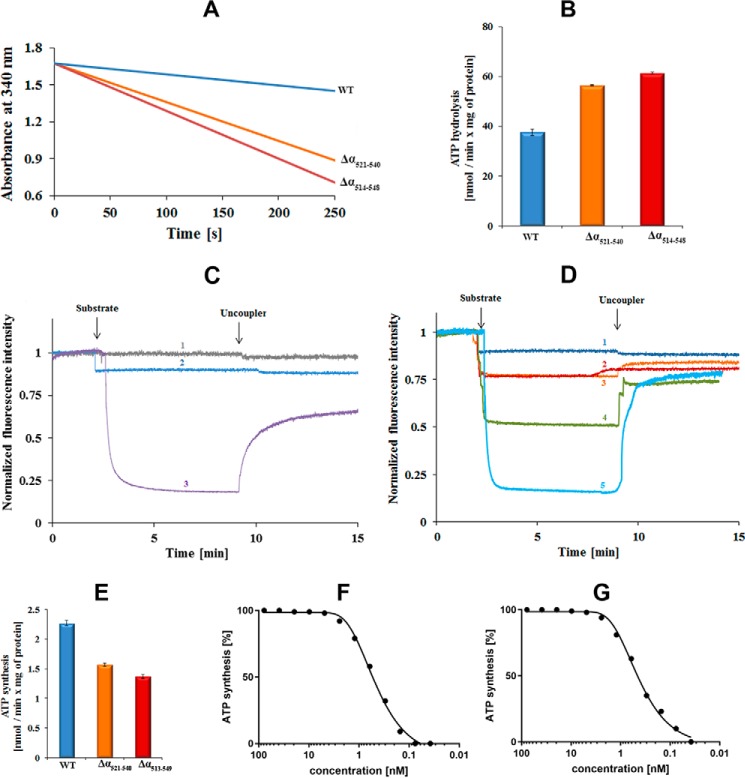
Figure 2.
Catalytic activities of M. smegmatis F-ATP synthase wt and mutant proteins. A, continuous ATPase activity of wt (blue) M. smegmatis F-ATP synthase, Δα(514–548) (red), and Δα(521–540) mutants (orange), respectively, using IMVs measured in the presence of type II NADH dehydrogenase inhibitor thioridazine (80 μm) and 2 mm MgATP. B, specific ATPase activity of wt, Δα(514–548), and Δα(521–540) mutant IMVs. Values are the mean of six determinations with two different IMV batches of wt and mutants. C and D, substrate driven proton-pumping in IMVs. C, M. smegmatis mc2 155 wt membrane vesicles were diluted to 0.18 mg/ml. Fluorescence quenching of ACMA by wt IMVs was studied after the addition of a substrate (2 mm ATP (blue, profile 2)) or 2 mm NADH (purple, profile 3). The uncoupler (SF6847) was added at the indicated time point to collapse the proton gradient. In the control experiment, buffer was added in place for substrate (gray, profile 1). D, fluorescence quenching of ACMA by IMVs of the Δα(514–548) (red, profile 2) and Δα(521–540) mutant (orange, profile 3) after addition of ATP in comparison to the wt IMVs (blue, profile 1) and the recently described Δγ(166–179) mutant (green, profile 4) (23). Profile 5 (light blue) reveals the quenching of IMVs of the Δ(α514–548) mutant in the presence of 2 mm NADH. Fluorescence quenching of ACMA with wt- and α-mutant IMVs was performed with four and two different batches of vesicles, respectively. E, ATP synthesis measured for wt (blue), Δα(514–548) (red) and Δα(521–540) mutant (orange) IMVs of M. smegmatis. Effect of increasing concentrations of bedaquiline on ATP synthesis using the M. smegmatis Δα(514–548) mutant (F) and wt IMVs (G).