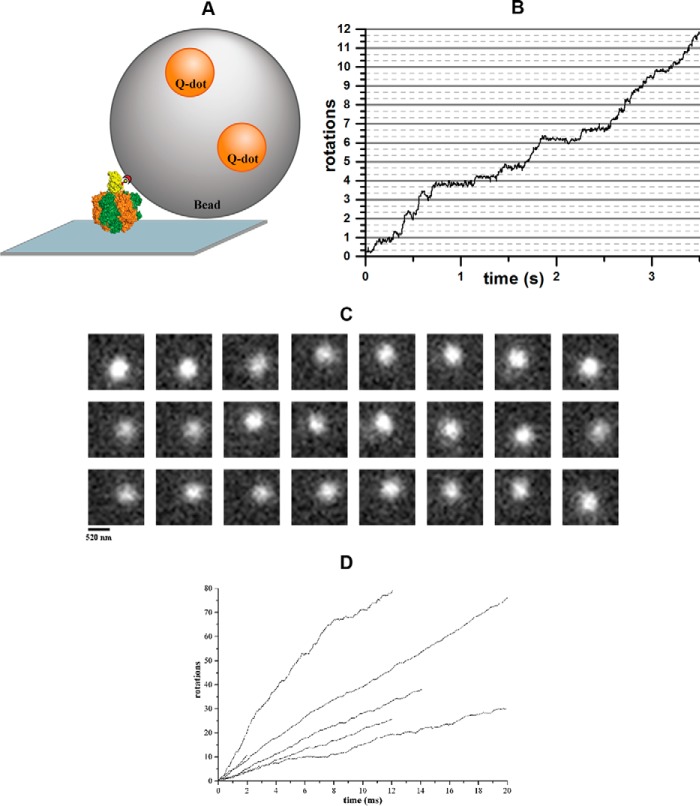
Figure 4.
Single-molecule measurements of rotating beads complexed with Gsα3β3γ or α3chiβ3γ. A, schematic model of the experimental setup for the single-molecule rotation assay of protein-bead complexes. The enzyme was fixed to a Ni-NTA-coated coverslide via its His tags, whereas the biotinylated cysteine at γ109 served to bind a streptavidin-coated bead (Ø = 0.3 μm) doped with biotinylated quantum dots. B, trajectory of a rotating Gsα3β3γ-bead complex with a rotational rate of 3.4 rotations per second. C, sequence of single video frames (30 ms per frame) showing the counterclockwise rotation of a single Gsα3β3γ-bead complex. Each frame has a resolution of 20 × 20 pixel with 65 nm/pixel. D, trajectories of rotating α3chiβ3γ-bead complexes, revealing that all protein-bead complexes are continuously rotating forward, i.e. counterclockwise, when active.