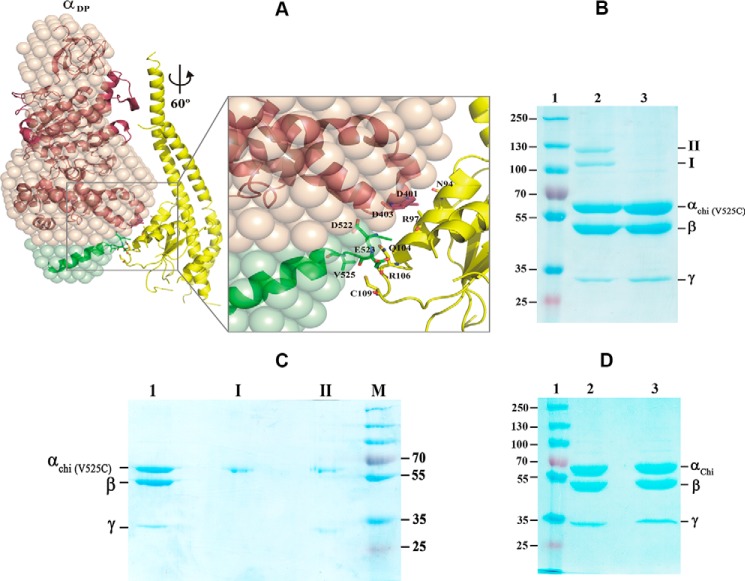
Figure 8.
Interaction of the C-terminal stretch of α with the rotary subunit γ. A, a structural model of the α3chiβ3γ complex was generated based on the G. stearothermophilus F1-ATPase structure (32) (PDB code 4XD7). See text for details. At 60° rotation, the α-helical residues 90AYNSNVLRLVYQT102 of the central globular domain of subunit γ (yellow) come closer to the so-called DELSEED region, composed of residues 396AQFGSDLDK404 of subunit α (brown), and the extended C-terminal domain of αchi (green). The polar residues Gln104 and Arg106 as well as Cys109 are in proximity to the extended C-terminal residues Asp522, Glu523, and Val525. B, Val525 was substituted by a cysteine in the mutant α3chiβ3γ complex mutant resulting in the (αchi-V525C)3β3γ. The mutant protein was applied on a 9% SDS gel in the absence (lane 2) or presence (lane 3) of DTT. Two cross-link products of the oxidized complex are marked I and II (lane 2). Lane 1, represents molecular weight standard proteins. C, the bands of product I and II of Fig. 9B were cut out, incubated in the same buffer with 20 mm DTT, before embedding in a second 9% SDS gel under reducing condition. Lanes 1–4, represent the (αchi-V525C)3β3γ, product I, product II, and a molecular mass standard, respectively. D, the (αchi-V525C)β3γ complex in the absence (lane 2) or presence (lane 3) of DTT was applied on a 9% SDS gel. Lane 1, molecular weight standard.