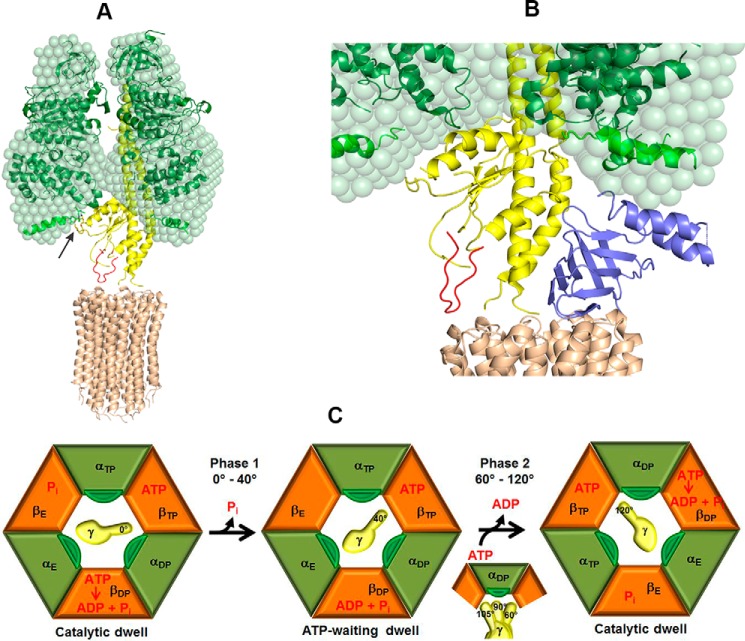
Figure 9.
Model of the interaction between subunits γ and αchi in α3chiβ3γ during rotation. A, the unique mycobacterial γ-loop (red), which is in the vicinity to the M. phlei c-ring (brown, PDB code 4V1G) (15), was inserted in the Gsγ (yellow). The arrow indicates the interaction between the 396AQFGSDLDK404 peptide (green) and the extra C-terminal domain of αchi (light green) with the α-helical peptide 90AYNSNVLRLVYQT102 of Gsγ. B, the proximity of the C-terminal helix of Mtϵ with the extended C-terminal stretch of αchi. Subunit ϵ (blue) inside the α3chiβ3γ complex was modeled based on the recently determined solution structure of Mtϵ (PDB code 5WY7), which was then superimposed onto the δ subunit of the bovine F1-ATPase. At 80° rotation of the central stalk, the compact C-terminal helix of Mtϵ might interact with the extended C-terminal domain of αchi on the way to form an extended conformation. C, subunits αchi, β with nucleotide occupancy, and γ with rotational position are shown in green, orange, and yellow, respectively, and labeled according to the native crystal structure (60). The extended C terminus of αchi is shown in dark green. Catalytic events are based on the model by Watanabe et al. (63). The state on the left shows the catalytic dwell. During phase 1 (see Fig. 5C) Pi release from βE triggers a 40° rotation of subunit γ, which leads the enzyme to adopt the ATP-waiting dwell. During phase 2, ATP binding and ADP release, accompanied by a change in the conformational states of the αchi and β subunits, causes subunit γ to further rotate by 80°. At a rotational position of 90° subunit γ is in close proximity to the extended C terminus of αchi.