Figure 1.
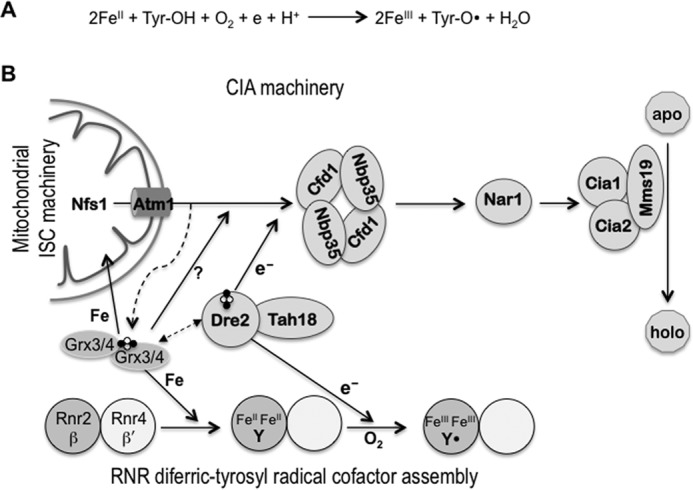
The relationship between the cofactor assembly in RNR and the pathways of iron-sulfur protein biogenesis in the mitochondria (ISC) and the cytosol (CIA) is shown. A, self-assembly of the RNR metallocofactor. The FeIII2-Y• cofactor can self-assemble in vitro from apo-β, FeII, and O2 in a four-electron reduction of O2 with the indicated stoichiometry. Of the four electrons required, two are from the two FeII ions serving as the metal ligand and one from a conserved tyrosine residue that is converted into a tyrosyl radical (Tyr-O•). The fourth electron, which can come from the excess of FeII in vitro, has to be supplied by an electron donor in vivo. B, connection between RNR radical cofactor assembly and cellular Fe-S protein biogenesis. Assembly of the FeIII2-Y• cofactor in Rnr2 (β) is facilitated by Rnr4 (β′), which stabilizes β in a conformation that allows iron binding. The monothiol glutaredoxins Grx3/4 function as [2Fe-2S]-GSH2-bridged dimers, and are required for several aspects of intracellular iron metabolism. They are involved in intracellular iron regulation (not depicted here), cytosolic Fe-S protein assembly, and in the formation of the FeIII2-Y• cofactor in RNR. The CIA pathway and RNR cofactor assembly share the electron donor complex Tah18-Dre2, which delivers electrons from NADPH to the scaffold protein complex Cfd1-Nbp35 in the CIA and to β in RNR. Genetic and physical interactions have been observed between Dre2 and Grx3/4 in both yeast and human cells (14, 29, 30), although the mechanistic basis of this interaction is unclear.
