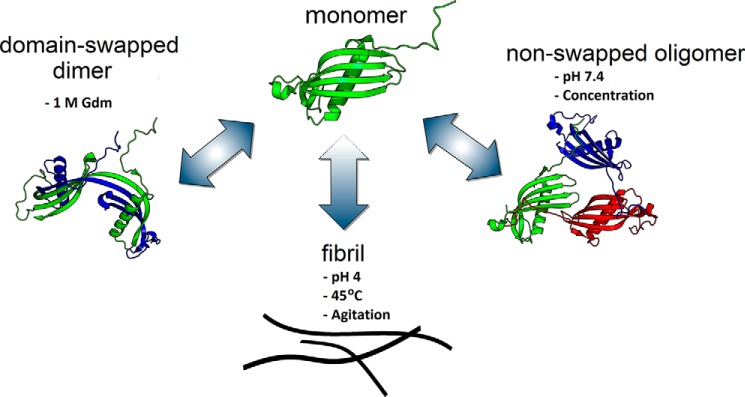
Figure 10.
Conceptual diagram of CysC aggregation pathways. CysC monomers self-associate by 3 distinct pathways. Mild chemical denaturation (1 m GdmHCl) supports rearrangement into domain-swapped dimers that are stable and no longer inhibit cysteine proteases. Mild concentrations during ultrafiltration or adsorption steps produces oligomers with similar secondary structure and enzyme inhibition properties as monomers. Agitation at acid pH and high temperature induces amyloid fibril formation. All 3 self-associated forms are fairly stable when returned to physiological solvent, which suggests that the monomer is a metastable state. Fibril growth proceeds through the monomer and does not require propagated domain-swapping.