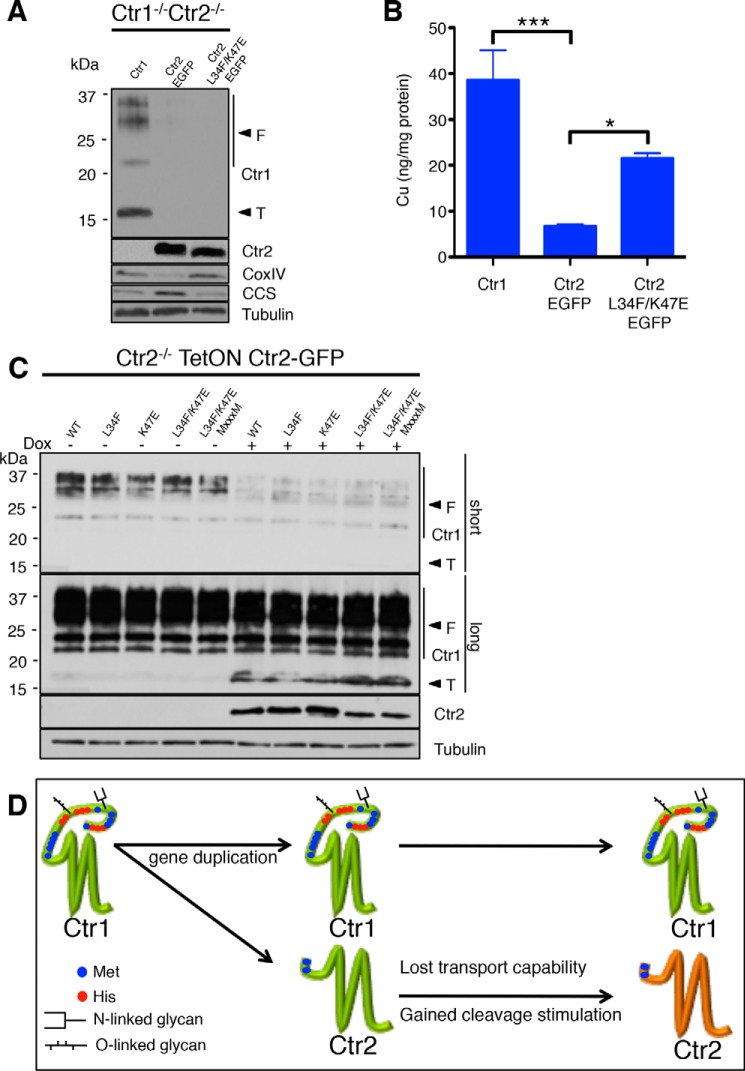
Figure 9.
Ctr2 transport-activating mutations increase bioavailable copper in mammalian cells while supporting Ctr1 ecto-domain cleavage. A, Ctr1−/−/Ctr2−/− MEFs containing the indicated gene were treated with 100 ng/ml doxycycline for 24 h, harvested, and immunoblotted with anti-Ctr1 (T, truncated; F, full-length), anti-GFP, anti-CoxIV, anti-CCS, and anti-tubulin antibodies. B, the same samples as in A were prepared for ICP-MS analysis of whole-cell copper levels. Data are presented as mean ± S.E. from four biological replicates. *, p ≤ 0.05; ***, p ≤ 0.001. C, Ctr2−/− MEFs containing the indicated gene were treated with 100 ng/ml doxycycline (Dox, +) or PBS (−) for 24 h, harvested, and immunoblotted. D, model for the appearance of Ctr2 during evolution. The single Ctr1 gene underwent a gene duplication to give rise to Ctr2, lacking the coding information for a copper-binding ecto-domain. The new Ctr2 gene then underwent at least two mutational events that led to a loss in the ability to transport Cu+ and a gain in the ability to stimulate the Cathepsin L-mediated cleavage of the Ctr1 ecto-domain.