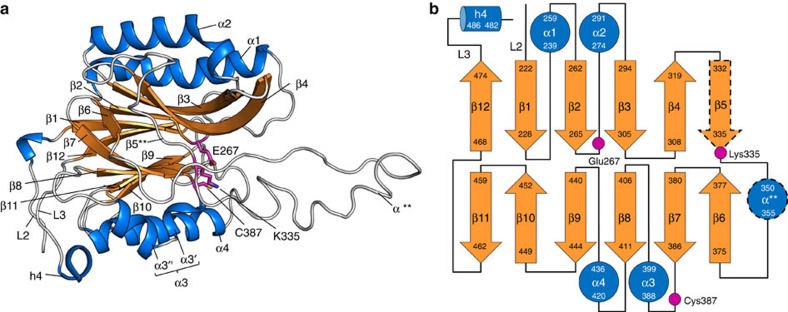
Figure 6. Nitrilase-like domain of LntEco.
(a) View from the membrane plane as in Fig. 3a. Colour coded by secondary structure to highlight the αββα sandwich feature of the domain. Catalytic triad residues are shown in stick representation. The asterisks in α** and β5** indicate that the α-helix and β-strand secondary structure elements form in some structures (α-helix found in: LntEco C387A, chain A; LntPae WT. β-strand found in: LntPae WT) but not in others (α-helix absent in: LntEco WT, chains A and B; LntEco C387A, chain B). (b) Schematic representation of the secondary structure elements in the nitrilase-like domain. Colour coding follows that used in a. Helix α3 consists of two small helices, α3′ and α3″. The dashed lines around α** and β5 indicate that these elements are formed in some structures but not in others as in a.