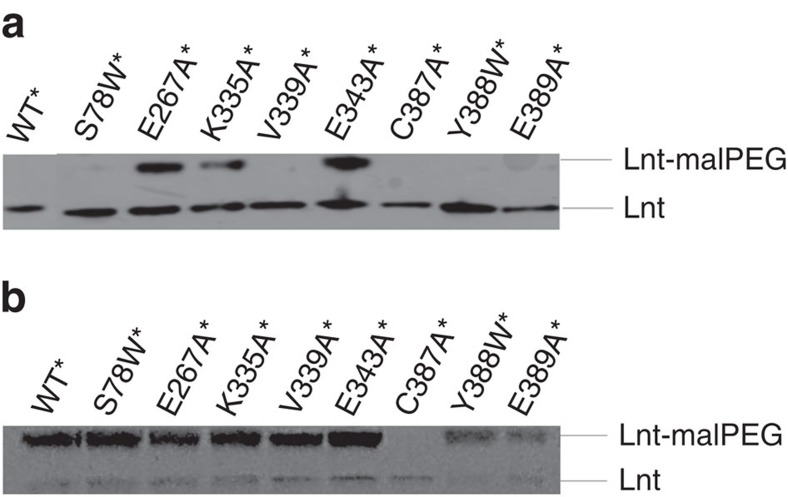
Figure 4. Effects of different mutated residues on the acylation activity of Lnt.
Eight key residues that caused dysfunction of Lnt in the in vivo assay were combined in the C23A/C62A variant of Lnt (that is, WT*) and were analysed by mal-PEG alkylation. Lnt (C23A/C62A/C387A), that is, C387A*, lacks any cysteine residue and was used as a negative control. Whole-cell lysates were treated without (a) or with (b) 1 M neutral hydroxylamine (which reduces all thioester bonds) and subsequently treated with mal-PEG. Samples were analysed by SDS-10% polyacrylamide gel electrophoresis followed by immunoblotting with antibodies against polyHis-tag. Alkylated (Lnt-mal-PEG) and non-alkylated forms of Lnt are indicated. Note that, variants E267A, K335A, E343A, C387A and E389A have been reported to be inactive using the same assay18.