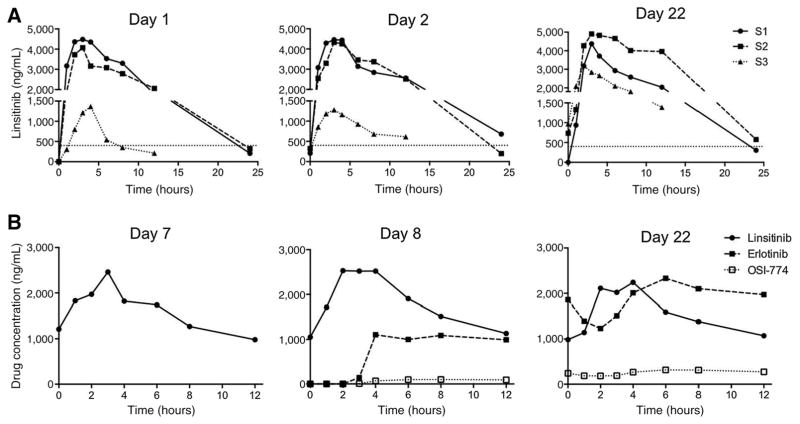
Figure 1.
Pharmacokinetic analysis of linsitinib, erlotinib and OSI-420. A, median plasma levels of linsitinib during day 1 (linsitinib alone), day 2 (first day of erlotinib dosing), and day 22 (day 1 cycle 2) in patients treated at the MTDs in the S1 (450/150 mg), S2 (400/100 mg), and S3 (150/150 mg) dose-escalation cohorts. Dotted line indicates 400 ng/mL concentration predicted from (15, 16) to be required for efficacy. B, median plasma levels of linsitinib, erlotinib, and OSI-420 during day 7 (linsitinib alone), day 8 (first day of erlotinib dosing), and day 22 (day 1, cycle 2) in NSCLC patients treated on the expansion cohort.