Figure 2. Scope of the enantioselective catalytic [2+2] cycloaddition of 2′-hydroxychalcones.
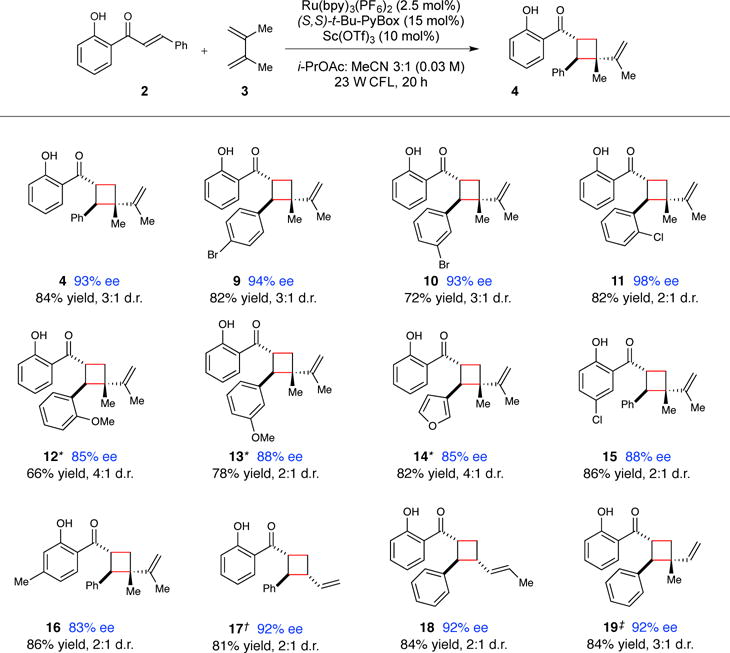
Data reflect the averaged isolated yields from two reproducible experiments. Diastereomer ratios were determined by 1H NMR analysis of the unpurified reaction mixture. Enantiomer ratios were determined using chiral SFC or HPLC analysis. See supplementary material for details. * Irradiation was conducted using a blue LED lamp instead of a 23 W CFL bulb, 2 h irradiation time. † 40 h irradiation time. ‡Isolated as a 6:1 mixture of regioisomers.
