Figure 1.
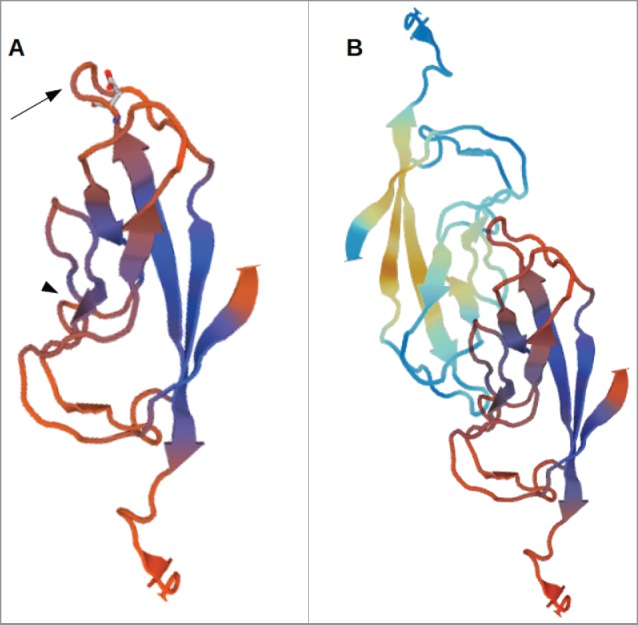
3D-model of the EC1-domain of LI-cadherin. (A) A model of the EC1-domain of LI-cadherin. The model is based on an energy minimisation of the primary sequence of LI-cadherin EC1 initiated by a template based on X-ray crystallography of E-cadherin EC1. Based on comparison with other cadherins (VE-cadherin, N-cadherin and E-cadherin) the loop between the β-sheets β6 and β7 (indicated by the arrow) was identified to be one binding site for LI-cadherin trans-interaction and an inhibitory peptide of the sequence VAALD was identified there. The aspartic acid of this peptide in the loop between β6 and β7 is indicated. (B) A model of the trans-interaction of 2 LI-cadherins. The orientation of the lower EC1 is identical to the single EC1 shown in (A). The loop between β6 and β7 interacts with corresponding amino acids in the vicinity of β4. This binding region close to β4 is indicated in (A) by the arrow-head.
