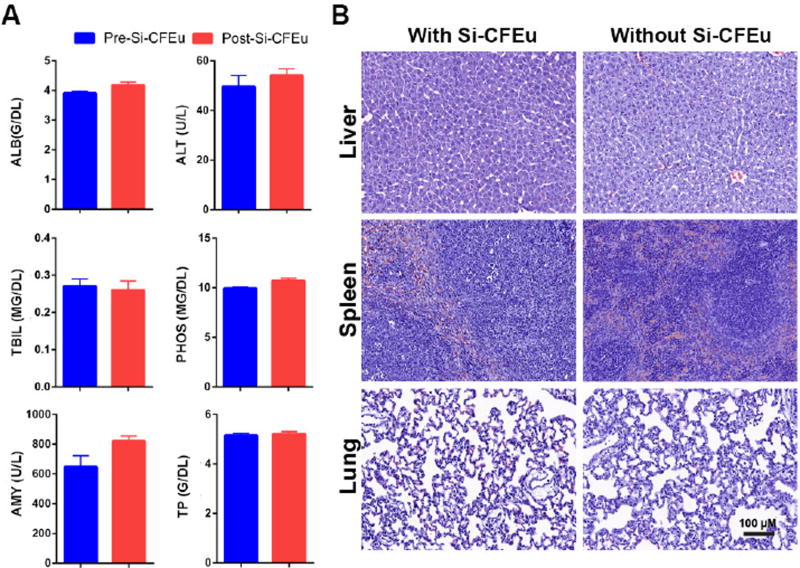
Fig. 11. Toxicity tests after Si-CFEu nanoparticle administration.
(A) Hepatic, renal and pancreatic functions (serum chemistry profile) were analyzed in rats pre-and post-Si-CFEu nanoparticles administration (ALB:Albumin, ALT: Alanine aminotransferase, TBIL: Total bilirubin, AMY: Amylase, PHOS: Phosphate, TP: Total protein). (B) H&E staining of liver, spleen and lungs of rats with and without administration of Si-CFEu nanoparticles. No abnormal pathology was detected in tissues in either group. Images were captured with 20× objective. (Pre-nanoparticle administration: 24 hours after 5 mg/kg LPS and prior to Si-CFEu nanoparticle administration).