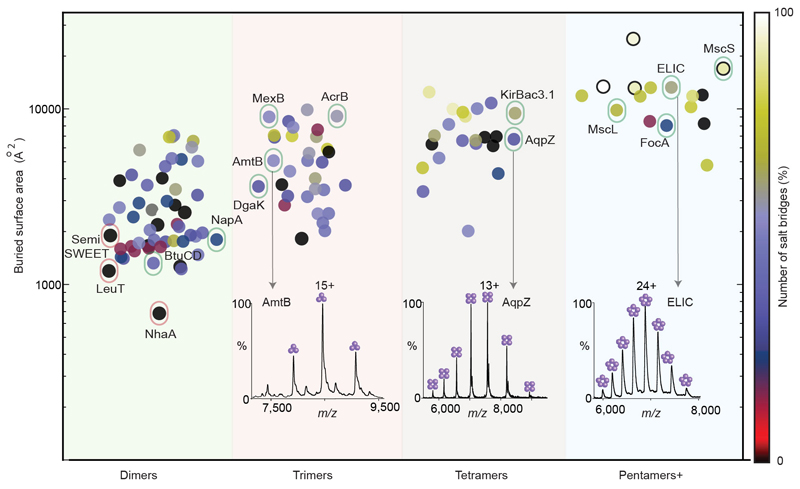
Figure 1. Plot of buried surface area and number of salt bridges for oligomeric α-helical membrane proteins and native mass spectra.
Protein oligomers are represented by circles color coded according to the number of salt bridges and are grouped by oligomeric state (pentamers+ oligomeric state ≥ 5). A random horizontal jitter has been applied to all points to aid visualisation. NhaA and LeuT (outlined in red) are two of the weakest oligomers having one of the lowest buried surface areas and no salt bridges. 12 proteins for which mass spectra have been recorded, are outlined in green. Illustrated are mass spectra of trimeric AmtB, tetrameric AqpZ and pentameric ELIC. A larger buried surface area than LeuT and NhaA, but absence of salt bridges, make SemiSWEET a relatively stronger dimer than LeuT and NhaA but weaker than the other 12 oligomeric proteins.