Figure 3.
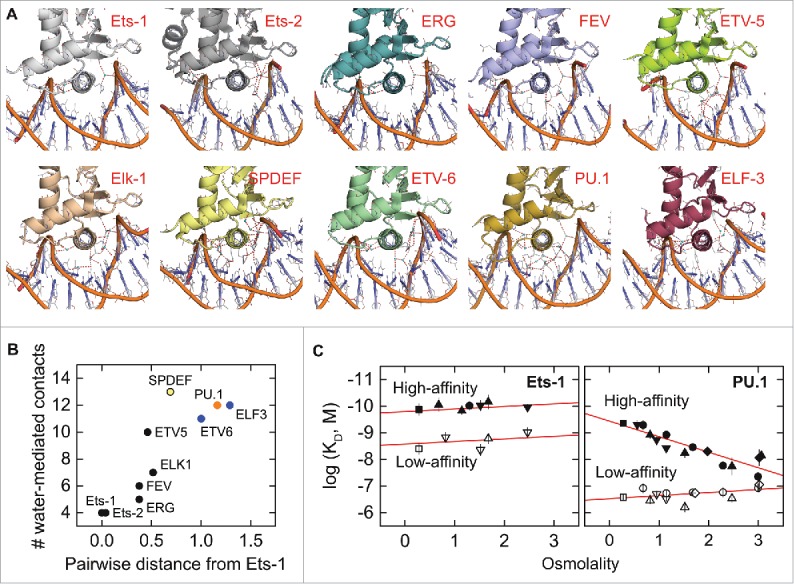
Crystallographic interfacial hydration correlates positively with phylogenetic relatedness among the ETS domains of paralogs. (A) Co-crystal structures of the ETS domains of nine ETS paralogs, oriented identically with the recognition helix perpendicular to the plane of the page. Water-mediated contacts are shown as cyan spheres, defined operationally as crystallographic water within hydrogen-bonding distance (red dashes, ≤ 3.4 Å) of a protein and DNA contact, or another interfacial water that meets this criterion. To avoid ambiguity, water-mediated contacts involving only three or more consecutive bridging water are not counted. Interfacial water density is weakly correlated with overall hydration of the asymmetric unit, and there is no significant difference in interfacial hydration density ( ± 1) between different biological units. (B) Relationship between crystallographic interfacial hydration and pairwise phylogenetic distance from Ets-1, chosen as reference. The primary sequences of the 28 human ETS paralogs were analyzed by ClustalW using the neighbor-joining method. The results were expressed as a distance matrix from whose elements are pairwise distances (number of substitutions per position). ETS paralogs are formally categorized into classes I–IV27 by color in order from black, blue, orange, to yellow. (C) Differential sensitivity to osmotic pressure in site-specific binding by the ETS domains of PU.1 and Ets-1 as reported by Wang et al.56 The measured in vitro affinity is expressed as the logarithm of the dissociation constant (KD). High- (solid symbols) and low-affinity DNA (open) refer to defined cognate (not nonspecific) sequences harboring the 5′-GGAA-3′ consensus. The different symbols refer to the set of physiologically compatible osmolytes used to exert osmotic stress. The osmotic insensitivity of Ets-1 is not modified by the presence of auto-inhibition.56
