Figure 1.
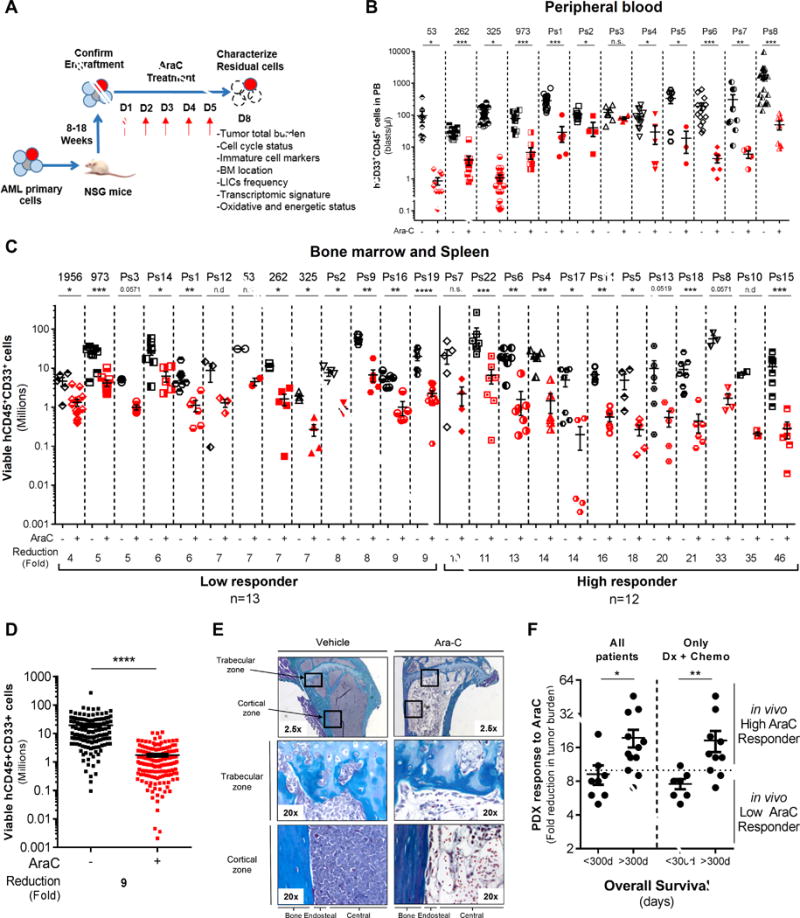
In vivo cytarabine (AraC) treatment induces a significant reduction of the total cell tumor burden in AML-engrafted mice. A, Schematic diagram of the chemotherapy regimen and schedule used to treat NSG-based PDX models with AraC. Peripheral blood engraftment was assessed between 8–18 weeks and mice were assigned to experimental groups of 4–10 mice with similar average engraftment per group. Mice were treated with vehicle (PBS) or 60mg/kg/d AraC given daily via IP injection for 5 days. Mice were sacrificed post-treatment at day 8 in order to characterize viable residual AML cells. B–C–D, Total number of human viable AML cells expressing CD45, CD33 and CD44 was analyzed and quantified using flow cytometry in AraC-treated AML-xenografted mice compared to PBS-treated AML-xenografted mice in peripheral blood (B; blasts per μL of mice blood) and in bone marrow and spleen (C; total cell tumor burden in Millions). Fold reduction of total cell tumor burden in AraC-treated mice compared with control-treated mice was calculated individually for each AML patient samples and in the entire PDX cohort. E, Goldner staining of bone marrow (tibia section at low/2.5x or high/20x magnification) shows engraftment and localization of AML cells at the cortical and trabecular region of the bone in vehicle (PBS)- and AraC-treated mice. F, Correlative analysis between the in vivo response to AraC using our PDX model and the overall survival of all matched AML patients or of those at diagnosis and that received intensive induction chemotherapy (Dx+Chemo). Graphs of mean ± sem. P values were determined by Mann-Whitney test. n.s., not significant. n.d., not determined. *, P≤0.05; **, P≤0.01; ***, P≤0.001.
