Figure 2.
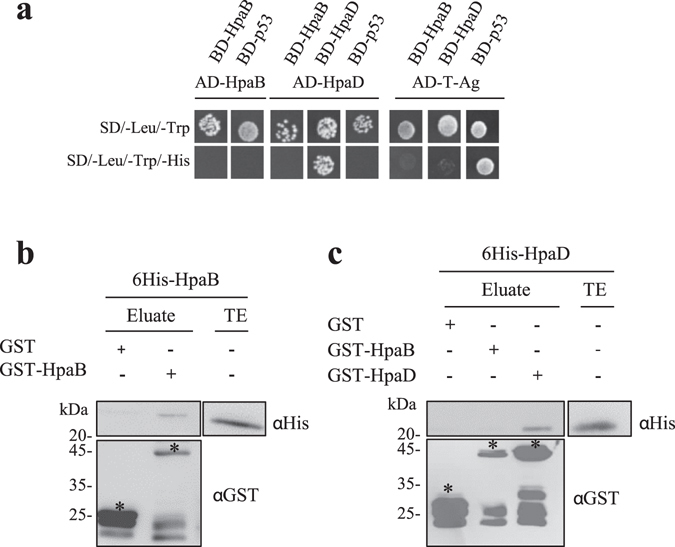
HpaB and HpaD self-interact but do not interact with each other. (a) Yeast cells were co-transformed by BD-HpaB or BD-HpaD and AD-HpaB or AD-HpaD. Double transformation and interaction were tested by plating yeasts on synthetic dropout medium lacking leucine and tryptophan (SD/–Leu/–Trp) and synthetic dropout medium lacking leucine, tryptophan and histidine (SD/–Leu/–Trp/–His), respectively. BD-p53 and AD-T-antigen were used as controls, together as a positive control (BD-p53/AD-T-Ag interaction is visualized with a yeast growth on minimal medium lacking histidine) and for tested interactors as negative controls. (b) Glutathione S-transferase (GST), GST-HpaB or GST-HpaD were immobilized on glutathione sepharose and incubated with an E. coli lysate containing 6His-HpaB or 6His-HpaD. Total cell lysates and eluted proteins were analysed using antibodies directed against GST and the 6His epitope. Bands corresponding to GST and GST fusion proteins are marked by asterisks. Lower bands represent degradation products. Two biological replicates were performed for (a) and (b) with similar results.
