Figure 5.
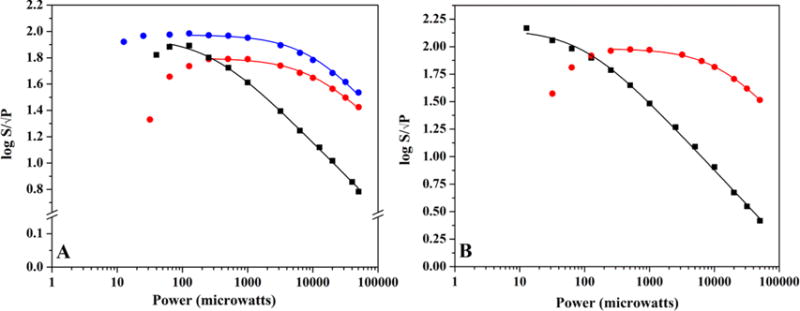
EPR power saturation curves of the g ≈ 2.0 signal in HydF. (A) The power saturation behavior of the g ≈ 2.0 signal in freshly purified HydF (111 μM protein at 2.50 ± 0.08 Fe/dimer) at 13 K (black) and 30 K (red). Also shown for comparative purposes is the power saturation behavior of the g ≈ 2.0 signal in as-isolated HydF (600 μM protein at 1.14 ± 0.08 Fe/dimer) at 30 K (blue). (B) The power saturation behavior of the g ≈ 2.0 signal in freshly purified photoreduced HydF (110 μM protein at 2.50 ± 0.08 Fe/dimer) that has undergone a thaw/freeze event (13 K, black; 30 K, red).
