Figure 6.
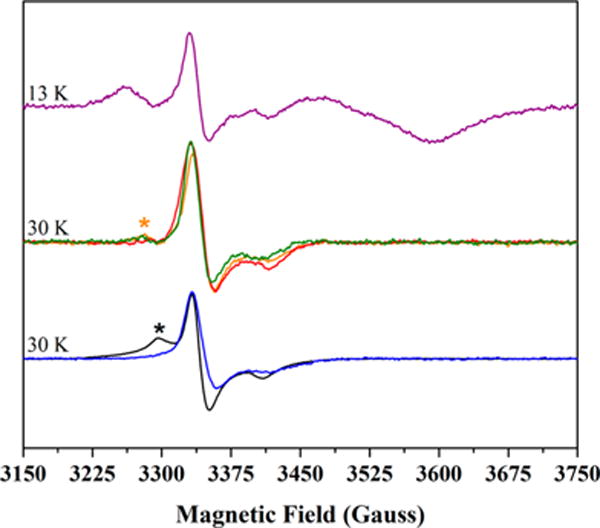
CW X-band EPR spectra of [2Fe-2S]+ cluster signals in HydF, PFL-AE, and HydE. The depicted spectra are as-isolated PFL-AE (1.68 mM protein with 2.70 ± 0.10 Fe/protein), black; as-reconstituted HydE (344 μM protein at 7.64 ± 0.10 Fe/protein), blue; freshly purified HydF (111 μM protein at 2.50 ± 0.08 Fe/dimer), green; as-isolated HydF (600 μM protein at 1.14 ± 0.08 Fe/dimer), orange; chemically reconstituted HydF (98 μM protein at 4.8 ± 0.8 Fe/dimer), red; photoreduced, freshly purified HydF (110 μM protein at 2.50 ± 0.08 Fe/dimer) following an anaerobic thaw/freeze event, purple. As this is a reduced sample, this latter spectrum also contains the axial [4Fe-4S]+ cluster signal, and this provides an internal frame of reference in the figure. Signal intensities for all data were arbitrarily normalized to scale spectra for direct comparison. Experimental temperature values for the various samples are provided in the figure; all spectra shown were collected at 1 mW power. The black asterisk denotes residual [3Fe-4S]+ content in the 30 K spectrum of PFL-AE (see Figures S6 and S10). The orange asterisk denotes a second [2Fe-2S]+ cluster signal that is most prevalent in the as-isolated HydF sample (refer to Figures S4, S8D, and S11D).
