Figure 3.
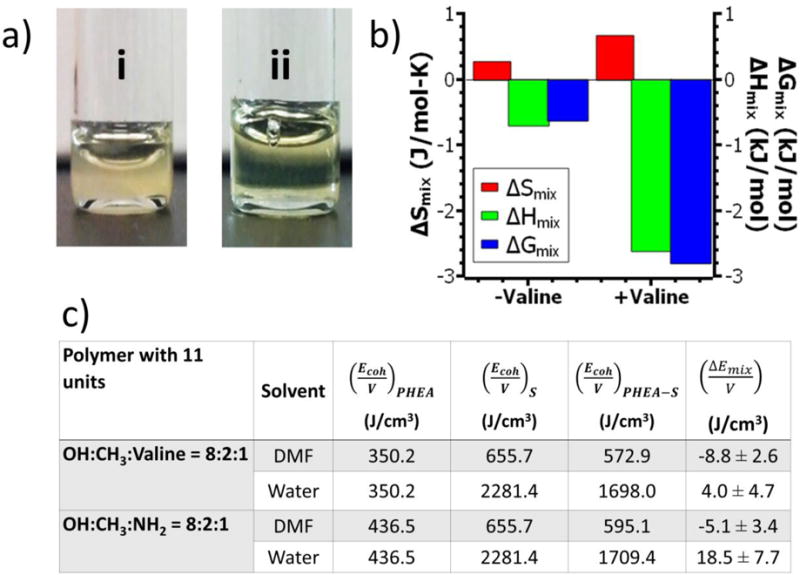
Effects of oligovaline on the solubility of PHEA in DMF. (a) Images of PHEA polymers dissolved in DMF at 30 mg/mL: (i) NH2-PHEA-C18 (DSC18 =20%); (ii) Oligovaline-PHEA-C18 (DSC18 =20%). (b) Changes in Gibb’s free energy of mixing (ΔGmix; blue), the heat of mixing (ΔHmix; green), and the entropy of mixing (ΔSmix; red) for oligovaline-PHEA-C18 (DSC18=20%; termed “+valine”) and NH2-PHEA-C18 (DSC18=20%; “-valine”). All values represent the average of values obtained at three different temperatures (−20, 0, or 25 °C). (c) The computational simulated energy of mixing per unit volume for the oligovaline-PHEA-C18 and the NH2-PHEA-C18 with 11 units dissolved in DMF or water at 30 vol%.
