Abstract
Self-rated health (SRH) is associated with morbidity and mortality in HIV-uninfected populations but is understudied in HIV. Substance use may affect SRH in addition to its deleterious effect on HIV disease. This analysis aimed to estimate SRH and substance use prevalence and evaluate factors associated with poor SRH among individuals in HIV care in Rio de Janeiro, Brazil. A convenience sample of HIV-infected adults completed one item of SRH, the Alcohol, Smoking and Substance Involvement Screening Test, and the Patient Health Questionnaire-2 (PHQ-2). Logistic regression models identified factors associated with poor SRH. Participants’ (n = 1029) median age was 42.9 years, 64.2% were male, and 54.5% were nonwhite. Poor SRH was reported by 19.5% and the use of alcohol, tobacco, marijuana, and crack/cocaine by 30.1, 19.5, 3.9, and 3.5%, respectively. Less than high school education (adjusted odds ratio [aOR] 1.54, 95% confidence interval [CI]: 1.08–2.20), lack of sexual activity in previous 12 months (aOR 1.53, 95% CI: 1.01–2.30), crack/cocaine use (aOR 3.82, 95% CI: 1.80–8.09), positive PHQ-2 screen (aOR 3.43, 95% CI: 2.09–5.62), and HIV-1 RNA ≥40 c/ml (aOR 2.51, 95% CI: 1.57–4.02) were significantly associated with poor SRH as identified by logistic regression analyses. Alcohol, marijuana, and sedative use were not significantly associated with poor SRH. These results emphasize the need for substance use and mental health screening and treatment in this population. Further research may elucidate the consequences of poor SRH on treatment adherence, morbidity, and mortality in HIV-infected individuals.
Keywords: Self-rated health, substance use, HIV/AIDS, self-assessment
Introduction
Self-rated health (SRH) assessments refer to questions that assess the respondent’s perception of his/her own health status. Different methods are used to evaluate SRH including validated questionnaires, like the Short Form Health Survey (SF-36), and the question, ‘How would you rate your health in general?’1 This question is particularly useful for its ease of implementation and is frequently used to assess national population health.1 The single-item of SRH became commonly used in the 1980s when an association between the single-item of SRH and mortality was demonstrated.1–3 In prior literature, associations with mortality persisted even when accounting for depression and physical function, but weakened when controlling for objective health measures.1 Poor SRH could therefore be associated with worse long-term health outcomes.
Accordingly, the number of studies searching for factors that predict good or poor SRH is increasing. Poor SRH is predicted by older age,4 race,5–7 low education,5,7–9 low income,5,8,9 socioeconomic disparity,10 low physical activity,5,8,9,11 comorbid disease and other objective health markers,1,3,8,12 and depressive symptoms.4,13 Studies from developed countries addressing gender differences have reported mixed results.3,7,9,14
Some of the aforementioned variables have been implicated in the syndemic theory of HIV, in which a co-occurring disease such as depression or an adverse psychosocial health condition, such as low socioeconomic status or substance use, may nurture or worsen the disease and related health outcomes, like SRH, in a given community.15 Though poor SRH in general populations has been consistently linked to syndemic conditions such as low education and income, the effects of substance use on SRH are less understood, likely due to variations in the studied populations, substance use definitions, and research methodologies. While the literature on the effects of illicit drug use on SRH is scarce, more research is published on tobacco and alcohol use. Results from a Canadian national health survey administered to nearly 14,000 adults reported that ever smoking was associated with a 74% increased chance of reporting poor SRH.9 The cumulative results of several studies on the association between alcohol and SRH are mixed3,8,16–19 with variations in study populations and methodologies.
Despite the relevance of syndemics to SRH and other poor long-term health outcomes in HIV, SRH is understudied in persons living with HIV/AIDS. One U.S. study of over 1700 HIV-infected adults found an association between poor SRH and death.20 Other international literature has shown that the absence of symptoms or medication side effects,21 high socioeconomic status, having a community-based network,22 and the absence of anxious and depressive feelings23 were associated with good SRH, but HIV-related markers like CD4+ T lymphocyte counts were not included in adjusted analyses. With respect to the effect of substance use on SRH in HIV-infected persons, Mrus et al.,24 using a two-item measure of SRH for a sample of 1649 adults, found that injection drug use history was associated with poor SRH. In another U.S. study of 184 adults, HIV-infected persons with alcohol use disorder that completed a 21-item variant of the Medical Outcome Survey: Health-Related Quality of Life (another measurement of SRH) reported lower health-related quality of life than those with either HIV or alcohol use disorder,25 thereby supporting the synergistic relationship between substance use and long-term health outcomes.
In the 2013 Brazilian National Health Survey, 33.9% rated their health as fair, bad, or very bad,26 similar to the proportion found (35%) in one cohort of Brazilian HIV-infected persons on antiretroviral therapy (ART).21 Moreover, 24% of adults reported consuming at least one alcoholic beverage per week and 15% used tobacco products daily or occasionally, but no information is available regarding current marijuana or crack/cocaine use. Apart from the aforementioned study of Brazilian HIV-infected individuals, there are no data on SRH or substance use prevalence in this population. Considering the relevance of SRH and substance use to long-term health outcomes and the current lack of information, we aimed to estimate the prevalence of poor SRH and its associated factors, including substance use, among HIV-infected adults in care in Rio de Janeiro, Brazil.
Methods
Study design
The STD/AIDS Clinical Research Laboratory at Instituto Nacional de Infectologia Evandro Chagas at Fundação Oswaldo Cruz (INI/FIOCRUZ), in Rio de Janeiro, Brazil, is a reference center for HIV treatment and research. As recommended by Brazil’s HIV treatment guidelines, patients have at least biannual appointments for follow-up care at INI.27 A cross-sectional study of a convenience sample of 1050 HIV-infected adults (≥18 years of age) who attended a routine appointment at INI between August 2013 and December 2015 was performed. The sole exclusion criterion was inability to provide informed consent. Trained nurses administered a structured interview that assessed SRH, depression, substance use, and sexual activity. These data were linked to INI’s HIV cohort database, a longitudinal database maintained since 1998 that includes demographic and clinical information, as previously described.28 Ethical approval was granted by the INI Institutional Review Board to the cross-sectional study (CAAE 17844113.2.0000.5262) as well as the parent cohort study (CAAE 0032.0.009. 000-10).
Outcome
SRH was measured by the question ‘How is your health?’ with possible answer choices of ‘Very bad,’ ‘Bad,’ and ‘Neither good nor bad’ categorized as ‘Poor SRH’ and ‘Good’ and ‘Very good’ categorized as ‘Good SRH,’ as previously dichotomized.26
Demographic, clinical, and behavioral variables
Sociodemographic factors were self-reported on the participant’s first clinic visit. ‘Sex’ was defined as sex at birth (male/female). Age at interview was defined as the difference in years between the questionnaire administration date and birth date, and a priori dichotomized so as to explore the effect of ‘older age’ for participants ≥50 years old, as suggested by Blanco et al.29 Educational level was dichotomized as no high school education versus ≥high school education. Race was categorized as ‘white,’ ‘black,’ or ‘mixed.’ Years with HIV diagnosis and years in HIV care were calculated as the difference in years between the interview date and the dates of the first positive HIV test and of the first clinic visit, respectively. The study instrument ascertained marital status, dichotomized as ‘single’ versus ‘married or living with partner,’ and sexual orientation, with response choices of ‘homosexual/gay,’ ‘heterosexual,’ and ‘bisexual’ dichotomized as ‘heterosexual’ versus ‘other.’
CD4+ T lymphocyte counts and HIV-1 RNA levels closest to the study administration date and within the prior 12 months were selected for analysis. Hepatitis B or C virus coinfection was defined as any record of a positive hepatitis B antigen test or hepatitis C antibody test. Metabolic disease was defined as meeting ≥1 of the following criteria by laboratory values taken within one year of the study administration: hypercholesterolemia (total cholesterol >239 mg/dl), hypertriglyceridemia (triglycerides >199 mg/dl), dyslipidemia (LDL >159 mg/dl or HDL <40 mg/dl), hypertension (diastolic blood pressure >100 mmHg), and diabetes (fasting blood glucose ≥126 mg/dl, random blood glucose ≥200 mg/dl, or hemoglobin A1c ≥6.5%). Lifetime history of an AIDS-defining illness was defined using the CDC 1993 criteria.30
Current tobacco, alcohol, marijuana, crack/cocaine, and nonprescription sedative use were assessed using the Portuguese validated version of the WHO’s Alcohol, Smoking and Substance Involvement Screening Test,31 specifically: ‘In the last 3 months, with what frequency did you use.…’ Possible answers were ‘Never,’ ‘1–2 times,’ ‘1–3 times/month,’ ‘1–4 times/week,’ and ‘5–7 days/week,’ dichotomized into ‘never’ and ‘any’ use. Binge drinking was assessed by the question ‘Have you ingested 5 or more alcoholic drinks in one occasion? One drink is one can of beer (300 mL) OR a glass of wine (120 mL) OR a shot of liquor (cachaça, vodka, whisky; 30 mL)’ with responses of ‘no, never,’ ‘yes, but not in the last 3 months,’ and ‘yes, in the last three months.’ This was dichotomized as ‘yes in the last three months’ or ‘no, not in the last 3 months.’
Depression screening used the Patient Health Questionnaire-2 (PHQ-2), validated in Brazilian primary health care populations,32 with the cutoff for a positive depression screen as a PHQ-2 value ≥3. The study instrument’s one item of sexual history asked participants to ‘mark all’ sexual partners that the participant had in the last 12 months: men, women, transsexuals, transvestites, and none. This was dichotomized into ‘any’ and ‘none.’
Statistical analyses
Categorical variables are described by their absolute and relative frequencies. Unadjusted logistic regression evaluated univariate associations between demographic, clinical, and behavioral variables and poor SRH. Stepwise backward logistic regression modeling was performed with all variables with p-values <0.10 in univariate modeling, removing terms of greatest non-significance until a final model was reached where all remaining variables presented a p-value ≤0.05. No variable was removed from the model if it changed the adjusted odds ratio (aOR) of another variable by more than 15%. To account for a large number of participants with missing CD4+ T lymphocyte counts (n = 442) and HIV-1 RNA levels (n = 429), a sensitivity analysis was conducted using the aforementioned statistical methods for participants with both CD4+ T lymphocyte counts and HIV-1 RNA levels (n = 576). Guided by previous findings,33 colinearity between 90-day crack/cocaine use and 90-day tobacco use was tested. When it was found, tobacco was excluded from regression models. Since colinearity between 90-day sedative and crack/cocaine use was found only in subset data, sedative use was also excluded from the regression model for the subset analysis. Age,4 sex at birth,3,7,9,14 and race5–7 were kept a priori in the final adjusted model because these variables were previously associated to SRH. Current CD4+ T lymphocyte count was kept in the final model despite borderline significance because it significantly changed the effect of HIV-1 RNA viral load. All statistical analyses were performed with R Statistical Software version 3.2.2.
Results
Of the 1050 study participants, 1029 were included for data completeness. Table 1 characterizes the overall study population. The participants were 64.2% male and 45.6% white, with a median age of 42.9 years (interquartile range 34.7, 50.6). About half of the population had some high school education or more and two-thirds identified as heterosexual. The median time since HIV diagnosis was 8.2 years, and the median time from initiation of HIV care was 6.1 years. Of the 587 participants with a CD4+ T lymphocyte count measured in the year prior to study administration, the median count was 599 cells/mm3.
Table 1.
Characteristics of study participants by self-rated health (SRH) status (good versus poor) and unadjusted odds ratios (OR) with 95% confidence intervals (95% CI), INI-Fiocruz from 2013 to 2015.
| Total | Good SRH | Poor SRH | OR (95% CI) | p-value | |
|---|---|---|---|---|---|
| Total | 1029 | 828 | 201 | ||
| Age (years) | 0.031 | ||||
| Median (IQR) | 42.9 (34.7, 50.6) | 42.6 (34.7, 50.2) | 44.3 (34.8, 52.3) | 1.01 (1, 1.02) | 0.128 |
| <50 | 751 (73) | 617 (74.5) | 134 (66.7) | REF | |
| ≥50 | 278 (27) | 211 (25.5) | 67 (33.3) | 1.46 (1.05, 2.04) | 0.025 |
| Sex at birth | 0.002 | ||||
| Male | 661 (64.2) | 551 (66.5) | 110 (54.7) | REF | |
| Female | 368 (35.8) | 277 (33.5) | 91 (45.3) | 1.65 (1.2, 2.25) | 0.002 |
| Racea | 0.068 | ||||
| White | 466 (45.6) | 389 (47.3) | 77 (38.3) | REF | |
| Black | 196 (19.2) | 151 (18.4) | 45 (22.4) | 1.51 (1, 2.28) | 0.052 |
| Mixed | 361 (35.3) | 282 (34.3) | 79 (39.3) | 1.42 (1, 2.01) | 0.051 |
| Educationa | <0.001 | ||||
| <High school | 479 (46.8) | 411 (49.9) | 68 (34.2) | REF | |
| ≥High school | 544 (53.2) | 413 (50.1) | 131 (65.8) | 1.92 (1.39, 2.65) | <0.001 |
| Sexual orientationa | 0.005 | ||||
| Homosexual/gay | 309 (30.5) | 269 (33) | 40 (20) | REF | |
| Heterosexual | 652 (64.3) | 502 (61.7) | 150 (75) | 2.01 (1.38, 2.94) | <0.001 |
| Bisexual | 53 (5.2) | 43 (5.3) | 10 (5) | 1.56 (0.73, 3.36) | 0.251 |
| Civil status | 0.575 | ||||
| Married or living with partner | 363 (35.3) | 296 (35.7) | 67 (33.3) | REF | |
| Single | 666 (64.7) | 532 (64.3) | 134 (66.7) | 1.11 (0.8, 1.54) | 0.52 |
| 12-month sexual activitya | 0.003 | ||||
| Yes | 830 (80.8) | 683 (82.7) | 147 (73.1) | REF | |
| None | 197 (19.2) | 143 (17.3) | 54 (26.9) | 1.75 (1.22, 2.52) | 0.002 |
| Years with HIV diagnosis | 0.431 | ||||
| Median (IQR) | 8.2 (4.1, 14.1) | 8.1 (4.1, 14.2) | 8.9 (4.4, 13.9) | 1 (0.98, 1.03) | 0.761 |
| Years in HIV care | 0.513 | ||||
| Median (IQR) | 6.1 (3.2, 10) | 6 (3.2, 10) | 6.4 (2.8, 9.9) | 0.99 (0.96, 1.02) | 0.562 |
| CD4 T lymphocyte count (cells/mm3) | <0.001 | ||||
| Median (IQR) | 599 (386, 828.5) | 610 (414, 852) | 468.5 (249.8, 713.5) | 1 (1, 1) | 0.003 |
| ≥500 | 358 (34.8) | 307 (37.1) | 51 (25.4) | REF | |
| <200 | 60 (5.8) | 38 (4.6) | 22 (10.9) | 3.49 (1.91, 6.37) | <0.001 |
| 200–500 | 169 (16.4) | 132 (15.9) | 37 (18.4) | 1.69 (1.05, 2.7) | 0.029 |
| Missing | 442 (43) | 351 (42.4) | 91 (45.3) | 1.56 (1.07, 2.27) | 0.02 |
| HIV-1 RNA level | <0.001 | ||||
| Undetectable | 423 (41.1) | 363 (43.8) | 60 (29.9) | REF | |
| Detectable | 177 (17.2) | 125 (15.1) | 52 (25.9) | 2.52 (1.65, 3.84) | <0.001 |
| Missing | 429 (41.7) | 340 (41.1) | 89 (44.3) | 1.58 (1.11, 2.27) | 0.012 |
| Time on ART (days) | 0.754 | ||||
| Median (IQR) | 6.1 (2.7, 12.8) | 6 (2.7, 13) | 7 (2.9, 12.4) | 1 (1, 1) | 0.948 |
| ≥90 days | 945 (91.8) | 762 (92) | 183 (91) | REF | |
| <90 days | 84 (8.2) | 66 (8) | 18 (9) | 1.14 (0.66, 1.96) | 0.648 |
| Lifetime AIDS-related disease | 0.008 | ||||
| None | 610 (59.3) | 508 (61.4) | 102 (50.7) | REF | |
| 1+ | 419 (40.7) | 320 (38.6) | 99 (49.3) | 1.54 (1.13, 2.1) | 0.006 |
| Hepatitis B infection | 0.798 | ||||
| No | 982 (95.4) | 789 (95.3) | 193 (96) | REF | |
| Yes | 47 (4.6) | 39 (4.7) | 8 (4) | 0.84 (0.39, 1.82) | 0.657 |
| Hepatitis C infection | 0.619 | ||||
| No | 953 (92.6) | 769 (92.9) | 184 (91.5) | REF | |
| Yes | 76 (7.4) | 59 (7.1) | 17 (8.5) | 1.2 (0.69, 2.11) | 0.518 |
| Metabolic variable | 0.581 | ||||
| No | 435 (42.3) | 354 (42.8) | 81 (40.3) | REF | |
| Yes | 594 (57.7) | 474 (57.2) | 120 (59.7) | 1.11 (0.81, 1.51) | 0.527 |
| PHQ-2a | <0.001 | ||||
| Negative | 938 (91.6) | 775 (94.1) | 163 (81.5) | REF | |
| Positive | 86 (8.4) | 49 (5.9) | 37 (18.5) | 3.59 (2.27, 5.68) | <0.001 |
| 90-day tobacco usea | 0.036 | ||||
| No | 826 (80.5) | 676 (81.8) | 150 (75) | REF | |
| Yes | 200 (19.5) | 150 (18.2) | 50 (25) | 1.5 (1.04, 2.17) | 0.029 |
| 90-day alcohol usea | 0.181 | ||||
| No | 716 (69.9) | 568 (68.8) | 148 (74) | REF | |
| Yes | 309 (30.1) | 257 (31.2) | 52 (26) | 0.78 (0.55, 1.1) | 0.155 |
| 90-day marijuana use | 0.134 | ||||
| No | 989 (96.1) | 800 (96.6) | 189 (94) | REF | |
| Yes | 40 (3.9) | 28 (3.4) | 12 (6) | 1.81 (0.91, 3.63) | 0.093 |
| 90-day crack/cocaine use | <0.001 | ||||
| No | 993 (96.5) | 810 (97.8) | 183 (91) | REF | |
| Yes | 36 (3.5) | 18 (2.2) | 18 (9) | 4.43 (2.26, 8.67) | <0.001 |
| 90-day sedative use | 0.059 | ||||
| No | 1008 (98) | 815 (98.4) | 193 (96) | REF | |
| Yes | 21 (2) | 13 (1.6) | 8 (4) | 2.6 (1.06, 6.36) | 0.036 |
| 90-day binge drinkinga | 0.776 | ||||
| No | 840 (82.6) | 677 (82.8) | 163 (81.9) | REF | |
| Yes | 177 (17.4) | 141 (17.2) | 36 (18.1) | 1.06 (0.71, 1.59) | 0.776 |
There were missing data for race (n = 6), education (n = 6), sexual orientation (n = 2), 12-month sexual activity (n = 2), 90-day tobacco (n = 3) and alcohol use (n = 4), and binge drinking (n = 12).
ART: antiretroviral therapy; IQR: interquartile range; PHQ-2: Patient Health Questionnaire-2.
In this population, 19.5% (n = 201) reported poor SRH and 80.5% (n = 828) reported good SRH with a distribution of very good 36% (n = 368), good 45% (n = 460), neither good nor bad 15% (n = 155), bad 3% (n = 36), and very bad 1% (n = 10). A total of 30.1 and 19.5% of study participants reported 90-day alcohol and tobacco use, respectively, while less than 5% reported 90-day marijuana, crack/cocaine use, or sedative use. Overall, 8.4% of participants were identified as having depressive symptoms per the PHQ-2 depression screen. Unadjusted analysis showed that age ≥50 years; female sex; less than high school education; heterosexual self-identification; absence of 12-month sexual activity; a lifetime diagnosis of an AIDS-defining illness; CD4+ T lymphocyte count <500 cells/mm3; detectable HIV-1 RNA level; and reported tobacco, crack/cocaine, or sedative use in the last 90 days were significantly associated (p <0.05) with poor SRH (Table 1).
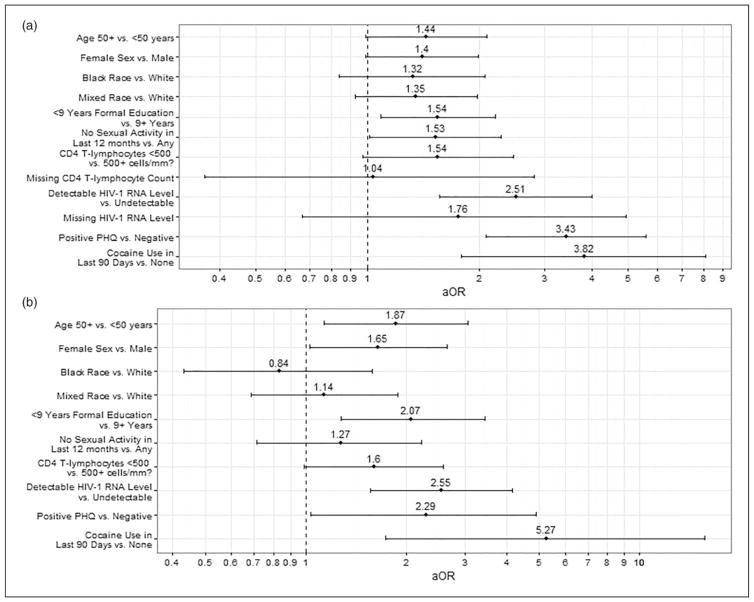
In adjusted analyses, those with poor SRH were less likely than those with good SRH to attend high school (p = 0.016) and have engaged in sexual activity in the last 12 months (p = 0.043). Persons with poor SRH were more likely to have a recent detectable HIV-1 RNA level (p <0.001), report crack/cocaine use in the last 90 days (p <0.001), and have a positive depression screen on the PHQ-2 (p <0.001) (Figure 1(a)). The effect sizes of recent crack/cocaine use (aOR = 3.82) and positive PHQ-2 screen (aOR = 3.43) were at least a third larger than the effect size of detectable HIV-1 RNA level (aOR = 2.51). Age ≥50 years (p = 0.057), female sex (p = 0.057), and a current CD4+ T lymphocyte count <500 cells/mm3 (p = 0.067) approached significance in the adjusted analysis (Figure 1(a)). The results of the sensitivity analysis of participants with complete data for both a recent CD4+ T lymphocyte count and a recent HIV-1 RNA level (n = 576) were not significantly different from those of the overall analysis (Figure 1(b), see Supplementary Material Table S1).
Figure 1.
Adjusted odds ratios (aOR) with 95% confidence intervals derived from multivariable regression analyses using statistically significant variables (p <0.1) associated with poor SRH from unadjusted logistic regression analysis. (a) All study participants (N = 1029) and (b) subset of study participants with recent CD4 count and HIV-1 RNA level (N = 576).
SRH: self-rated health.
Discussion
Of individuals in HIV care at INI, 80.5% reported good SRH. The high prevalence of good SRH compared to that found in the Brazilian general population (66.1%)26 may reflect the fact that our sample was recruited at a multidisciplinary care center and is likely to have better access to care than the general population. In addition, participants may be primed to respond to SRH questions from the perspective of an HIV-infected individual, using other HIV-infected peers or their own prior health experiences as a frame of reference, and, consequently, may find themselves to be in comparatively good health.1 Our prevalence of good SRH was also higher than that of a multicenter Brazilian cohort of HIV-infected persons (65%) by 15%,21 possibly because there are unmeasured factors related to health services at play and because current ART regimens are better tolerated than those available in 2008 when the study was conducted. Poor SRH was associated with lower schooling, no reported sexual activity in the last 12 months, positive 90-day recall of crack/cocaine use, a positive PHQ-2 screen, and HIV-1 RNA levels ≥40 copies/ml.
The prevalence of 90-day alcohol use (30.1%), marijuana use (3.9%), and crack/cocaine use (3.1%) was similar to a one-week prevalence found in the same cohort,34 although 90-day tobacco use (19.5%) was smaller than that of 12-month use (29%),33 as expected. All estimates, however, were lower than that of U.S. HIV-infected cohorts, where 50–70% report smoking,35 53% report drinking in the past month,36 and 24 and 9% report 90-day marijuana and crack/cocaine use, respectively.37 These studies used computer-assisted questionnaires which may confer less social desirability bias than a nurse-administered questionnaire such as that of our study.38 Moreover, these lower prevalences may reflect the difficulty in reaching and linking substance users with HIV care.39,40
Both a positive depression screen and current crack/ cocaine use showed the largest effect sizes on poor SRH in our analysis, roughly a third larger than the most strongly associated clinical variable, a detectable HIV-1 RNA level. Depression is a significant contributor to SRH, not only because of its effects on objective health measures,41 but also because it distorts self-perception.1,3 Hence, it is important to screen for depression in HIV-infected persons. The association between crack/cocaine and poor SRH adds to a small, conflicting body of literature in which one study found that crack/cocaine smokers were more likely to report poor SRH,16 while another U.S. survey of roughly 19,000 adults aged 50 or older did not find an association (though the analysis was limited by small sample of crack/cocaine users).17 In our analysis, this association presented a large effect size even after controlling for standard measures of HIV disease severity implying that crack/cocaine use may affect other non-HIV related clinical variables or the process of self-evaluation of health. For example, crack users may see crack addiction as worse for their health than alcohol or tobacco addiction16 and consequently evaluate their SRH as poor. Adding to the conflicting body of literature, there was no association between alcohol use and SRH. In sum, the data suggest that substance use screening should be a part of routine HIV care.
The association between poor SRH and low levels of education may reflect limited access to resources, like information about health-promoting behaviors and social support networks, or a conception of health rooted in a weaker base of clinical information.42,43 Not only was the proportion of study participants reporting sexual activity in the last year (80.5%) similar to that of Brazilians aged 15–64 (77.3%), but the breakdown by gender was also similar: 81% of men and 73.7% of women in the national population, and 85.2% of men and 73% of women in our study population reported 12-month sexual activity.44 Those with no recent sexual activity could be mentally distressed or too physically ill,45 or may suffer from decreased libido from chronic illness,46 all of which may thereby affect SRH. This adds another dimension to the importance of asking HIV-infected persons about their sex lives or lack thereof, as it may have a negative impact on SRH. HIV-related measures, CD4+ T lymphocyte count and HIV-RNA level, affected SRH as previously described.1,24 However, a limitation of this study was the large number of missing laboratory information. This could have excluded a population that are poorly linked to care and therefore may be sicker with poorer SRH; however, the sensitivity analyses did not yield major differences in the demographics (see Supplementary Material for details, Table S1) nor the multivariable analyses (Figure 1) between those with complete laboratory information and those without. One notable difference was that the effect size of a positive PHQ-2 screen decreased when participants with missing information were removed from the analysis, suggesting that PHQ-2 is a weaker correlate with SRH in the presence of clinical information (Figure 1(b)). Another limitation of the study was the inclusion only of participants who were attending scheduled out-patient appointments, who were more likely to be female, non-white, and have less education than the eligible population that did not complete the study (Table S2). Though patients linked to care may be expected to have higher CD4+ T lymphocyte counts and lower HIV-1 RNA levels, and therefore report better SRH, there were no differences between these two populations on these measures (Table S2). However, HIV-infected individuals that did not attend their outpatient appointments may be missing their appointments due to other social and behavioral variables that may negatively influence their SRH, such as drug and problematic alcohol use. In fact, the prevalence of drug and alcohol use was too small to stratify into occasional and heavy users. It is possible that heavy users would be more likely to report poor SRH than occasional users. Additionally, this study did not address other chronic health diseases that may adversely affect SRH, such as cancer and heart disease. Given the cross-sectional design of the study, causality may not be inferred and, although results relating to SRH are similar to other Brazilian estimates, given the non-probabilistic nature of the sample, results may not be generalizable to all individuals in care for HIV in the country.
This study has identified that individuals with lower education, with positive screening for depression, and cocaine users have an increased chance of reporting poor SRH. Considering that the screen for SRH (measured by a single question) is easy to implement in clinical settings, this question may be useful to screen for psychological and social distress in primary/secondary health services that are not focused on these problems. Given that the previously reported association between SRH and mortality has implications on the population level, SRH would be important to evaluate in future research.1–3
Conclusions
The proportion of HIV-infected adults in care that report poor SRH was lower in our sample than in other studies of HIV-infected Brazilians and the Brazilian general population, a result that deserves further investigation. Since participants presenting a positive screen for depression and use crack/cocaine were more likely to report poor SRH, it is important to incorporate mental health and substance use screening and treatment into the care of HIV-infected persons. Additional research is needed to elucidate the effect SRH may have on treatment adherence, morbidity, and mortality.
Supplementary Material
Acknowledgments
We would like to thank the participants enrolled in the INI HIV/AIDS program and the dedicated staff at INI.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by the National Institutes of Health grants R25 MH087222 (South American Program in HIV Research) and K23 AI110532 in addition to the NIH-funded Caribbean, Central and South America network for HIV epidemiology (CCASAnet), a member cohort of the International Epidemiologic Databases to Evaluate AIDS (leDEA, U01AI069923), and the Conselho Nacional de Desenvolvimento Cientifico e Tecnologico (CNPq, 476024/ 2013-7).
Footnotes
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval
All procedures performed in this study, including informed consent obtained from all individual participants included in the study, were in accordance with the ethical standards of the institutional and national research committees and with the 1964 Helsinki declaration and its later amendments.
References
- 1.Jylhä M. What is self-rated health and why does it predict mortality? Towards a unified conceptual model. Soc Sci Med. 2009;69:307–316. doi: 10.1016/j.socscimed.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 2.DeSalvo KB, Bloser N, Reynolds K, et al. Mortality prediction with a single general self-rated health question: a meta-analysis. J Gen Intern Med. 2006;21:267–275. doi: 10.1111/j.1525-1497.2005.00291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Idler EL, Benyamini Y. Self-rated health and mortality: a review of twenty-seven community studies. J Health Soc Behav. 1997;38:21–37. [PubMed] [Google Scholar]
- 4.Pinquart M. Correlates of subjective health in older adults: a meta-analysis. Psychol Aging. 2001;16:414–426. doi: 10.1037//0882-7974.16.3.414. [DOI] [PubMed] [Google Scholar]
- 5.Lorraine PJ, Hammock RL, Blanton JM. Predictors of self-rated health status among Texas residents. Prev Chronic Dis. 2005;2:A12. [PMC free article] [PubMed] [Google Scholar]
- 6.Cagney KA, Browning CR, Wen M. Racial disparities in self-rated health at older ages: what difference does the neighborhood make? J Gerontol Ser B Psychol Sci Soc Sci. 2005;60:S181–S190. doi: 10.1093/geronb/60.4.s181. [DOI] [PubMed] [Google Scholar]
- 7.Franks P, Gold MR, Fiscella K. Sociodemographics, self-rated health, and mortality in the US. Soc Sci Med. 2003;56:2505–2514. doi: 10.1016/s0277-9536(02)00281-2. [DOI] [PubMed] [Google Scholar]
- 8.Wu S, Wang R, Zhao Y, et al. The relationship between self-rated health and objective health status: a population-based study. BMC Public Health. 2013;13:9. doi: 10.1186/1471-2458-13-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cott CA, Gignac MA, Badley EM. Determinants of self rated health for Canadians with chronic disease and disability. J Epidemiol Community Health. 1999;53:731–736. doi: 10.1136/jech.53.11.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kondo N, Sembajwe G, Kawachi I, et al. Income inequality, mortality, and self rated health: meta-analysis of multilevel studies. BMJ. 2009;339:b4471. doi: 10.1136/bmj.b4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benyamini Y. Why does self-rated health predict mortality? An update on current knowledge and a research agenda for psychologists. Psychol Health. 2011;26:1407–1413. doi: 10.1080/08870446.2011.621703. [DOI] [PubMed] [Google Scholar]
- 12.Jylhä M, Volpato S, Guralnik JM. Self-rated health showed a graded association with frequently used biomarkers in a large population sample. J Clin Epidemiol. 2006;59:465–471. doi: 10.1016/j.jclinepi.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 13.Chang-Quan H, Xue-Mei Z, Bi-Rong D, et al. Health status and risk for depression among the elderly: a meta-analysis of published literature. Age Ageing. 2010;39:23–30. doi: 10.1093/ageing/afp187. [DOI] [PubMed] [Google Scholar]
- 14.Szwarcwald CL, de Souza PRB, Júnior, Esteves MAP, et al. Sociodemographic determinants of self-rated health in Brazil. Cad Saude Publica. 2005;21:S54–S64. doi: 10.1590/s0102-311x2005000700007. [DOI] [PubMed] [Google Scholar]
- 15.Singer M, Clair S. Syndemics and public health: reconceptualizing disease in bio-social context. Med Anthropol Q. 2003;17:423–441. doi: 10.1525/maq.2003.17.4.423. [DOI] [PubMed] [Google Scholar]
- 16.Falck RS, Wang J, Carlson RG, et al. Crack-cocaine use and health status as defined by the SF-36. Addict Behav. 2000;4:579–584. doi: 10.1016/s0306-4603(99)00040-4. [DOI] [PubMed] [Google Scholar]
- 17.Blazer DG, Wu L-T. The epidemiology of substance use and disorders among middle aged and elderly community adults: national survey on drug use and health. Am J Geriatr Psychiatry. 2009;17:237–245. doi: 10.1097/JGP.0b013e318190b8ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frisher M, Mendonça M, Shelton N, et al. Is alcohol consumption in older adults associated with poor self-rated health? Cross-sectional and longitudinal analyses from the English Longitudinal Study of Ageing. BMC Public Health. 2015;15:703. doi: 10.1186/s12889-015-1993-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Valencia-Martín JL, Galán I, Rodríguez-Artalejo F. Alcohol and self-rated health in a Mediterranean country: the role of average volume, drinking pattern, and alcohol dependence. Alcohol Clin Exp Res. 2009;33:240–246. doi: 10.1111/j.1530-0277.2008.00826.x. [DOI] [PubMed] [Google Scholar]
- 20.Fleishman JA, Crystal S. Functional status transitions and survival in HIV disease: evidence from the AIDS costs and service utilization survey. Source Med Care. 1998;36:533–543. doi: 10.1097/00005650-199804000-00009. [DOI] [PubMed] [Google Scholar]
- 21.Souza PRB, Junior, Borges De Souza PR, Junior, Szwarcwald CL, et al. Self-rated health by HIV-infected individuals undergoing antiretroviral therapy in Brazil. Cadernos De Saude Publica. 2011;27:S56–S66. doi: 10.1590/s0102-311x2011001300007. [DOI] [PubMed] [Google Scholar]
- 22.Dageid W, Grønlie AA. The associations between resilience, social capital and self-rated health among HIV-positive South Africans. J Health Psychol. 2015;20:1463–1473. doi: 10.1177/1359105313513623. [DOI] [PubMed] [Google Scholar]
- 23.Sun W, Wu M, Qu P, et al. Psychological well-being of people living with HIV/AIDS under the new epidemic characteristics in China and the risk factors: a population-based study. Int J Infect Dis. 2014;28:147–152. doi: 10.1016/j.ijid.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 24.Mrus JM, Schackman BR, Wu AW, et al. Variations in self-rated health among patients with HIV infection. Qual Life Res. 2006;15:503–514. doi: 10.1007/s11136-005-1946-4. [DOI] [PubMed] [Google Scholar]
- 25.Rosenbloom MJ, Sullivan EV, Sassoon SA, et al. Alcoholism, HIV infection, and their comorbidity: factors affecting self-rated health-related quality of life. J Stud Alcohol Drugs. 2007;68:115–125. doi: 10.15288/jsad.2007.68.115. [DOI] [PubMed] [Google Scholar]
- 26.IBGE IB de G e, Estatística. Pesquisa Nacional de Saúde 2013: percepção do estado de saúde, estilos de vida e doenças crônicas. http://biblioteca.ibge.gov.br/visualizacao/livros/liv91110.pdf.
- 27.Brasil MDS. [accessed 23 January 2017];Protocolo clínico e diretrizes terapêuticas para manejo da infeccção pelo hiv em adultos. 2013 http://www.aids.gov.br/sites/default/files/anexos/publicacao/2013/55308/protocolofinal_31_7_2015_pdf_31327.pdf.
- 28.Grinsztejn B, Veloso VG, Friedman RK, et al. Early mortality and cause of deaths in patients using HAART in Brazil and the United States. AIDS. 2009;23:2107–2114. doi: 10.1097/QAD.0b013e32832ec494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blanco JR, Jarrín I, Vallejo M, et al. Definition of advanced age in HIV infection: looking for an age cutoff. AIDS Res Hum Retroviruses. 2012;28:1000–1006. doi: 10.1089/aid.2011.0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.CDC. 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep. 1992;41:1–19. [PubMed] [Google Scholar]
- 31.Henrique IFS, De Micheli D, De Lacerda RB, et al. Validacao da Versao Brasileira do Teste de Triagem do Envolvimento com Alcool, Cigarro e Outras Substancias (ASSIST) Rev Assoc Med Bras. 2004;50:199–206. doi: 10.1590/s0104-42302004000200039. [DOI] [PubMed] [Google Scholar]
- 32.de Lima Osório F, Vilela Mendes A, Crippa JA, et al. Study of the discriminative validity of the PHQ-9 and PHQ-2 in a sample of Brazilian women in the context of primary health care. Perspect Psychiatr Care. 2009;45:216–227. doi: 10.1111/j.1744-6163.2009.00224.x. [DOI] [PubMed] [Google Scholar]
- 33.Torres TS, Luz PM, Derrico M, et al. Factors associated with tobacco smoking and cessation among HIV-Infected individuals under care in Rio de Janeiro, Brazil. PLoS One. 2014;9:1–15. doi: 10.1371/journal.pone.0115900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Boni RB, Shepherd BE, Grinsztejn B, et al. Substance use and adherence among people living with HIV/AIDS receiving cART in Latin America. AIDS Behav. 2016;11:2692–2699. doi: 10.1007/s10461-016-1398-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nahvi S, Cooperman NA. Review: the need for smoking cessation among HIV-positive smokers. AIDS Educ Prev. 2009;21:14–27. doi: 10.1521/aeap.2009.21.3_supp.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Galvan FH, Bing EG, Fleishman JA, et al. The prevalence of alcohol consumption and heavy drinking among people with HIV in the United States: results from the HIV Cost and Services Utilization Study. J Stud Alcohol. 2002;63:179–186. doi: 10.15288/jsa.2002.63.179. [DOI] [PubMed] [Google Scholar]
- 37.Mimiaga MJ, Reisner SL, Grasso C, et al. Substance use among HIV-infected patients engaged in primary care in the United States: findings from the Centers for AIDS Research Network of Integrated Clinical Systems cohort. Am J Public Health. 2013;103:1457–1467. doi: 10.2105/AJPH.2012.301162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krumpal I. Determinants of social desirability bias in sensitive surveys: a literature review. Qual Quant. 2013;47:2025–2047. [Google Scholar]
- 39.Bell C, Metsch LR, Vogenthaler N, et al. Never in care: characteristics of HIV-infected crack cocaine users in 2 US cities who have never been to outpatient HIV care. J Acquir Immune Defic Syndr. 2010;54:376–380. doi: 10.1097/QAI.0b013e3181d01d31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tobias CR, Cunningham W, Cabral HD, et al. Living with HIV but without medical care: barriers to engagement. AIDS Patient Care STDS. 2007;21:426–434. doi: 10.1089/apc.2006.0138. [DOI] [PubMed] [Google Scholar]
- 41.Leserman J. Role of depression, stress, and trauma in HIV disease progression. Psychosom Med. 2008;70:539–545. doi: 10.1097/PSY.0b013e3181777a5f. [DOI] [PubMed] [Google Scholar]
- 42.Kawachi I, Kennedy BP, Glass R. Social capital and self-rated health: a contextual analysis. Am J Public Health. 1999;89:1187–1193. doi: 10.2105/ajph.89.8.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dowd JB, Zajacova A. Does the predictive power of self-rated health for subsequent mortality risk vary by socioeconomic status in the US? Int J Epidemiol. 2007;36:1214–1221. doi: 10.1093/ije/dym214. [DOI] [PubMed] [Google Scholar]
- 44.Brasil MDS. [accessed 23 January 2017];Pesquisa de Conhecimentos, Atitudes e Práticas na População Brasileira. 2011 http://bvsms.saude.gov.br/bvs/publicacoes/pesquisa_conhecimentos_atitudes_praticas_populacao_brasileira.pdf.
- 45.Maticka-Tyndale E, Adam BD, Cohen J. Sexual desire and practice among people living with HIV and using combination anti-retroviral therapies. Can J Hum Sex. 2002;11:33–41. [Google Scholar]
- 46.Basson R, Rees P, Wang R, et al. Sexual function in chronic illness. J Sex Med. 2010;7:374–388. doi: 10.1111/j.1743-6109.2009.01621.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.