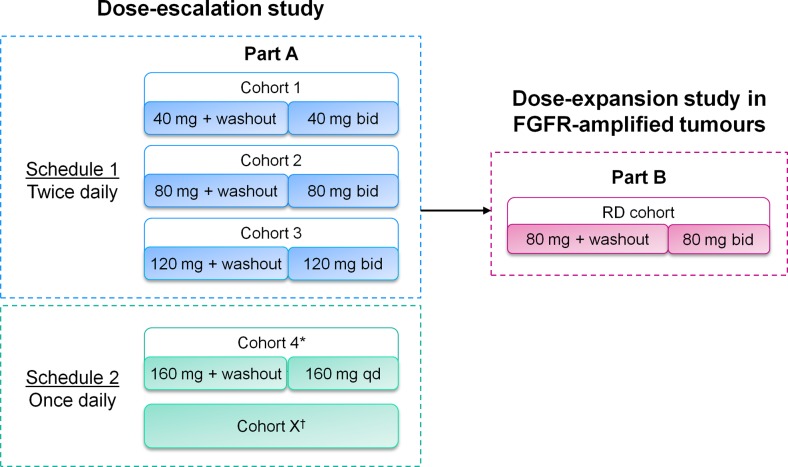
Fig. 1.
AZD4547 Japanese Phase I study design. Part A was a dose-escalation study with a 5- to 10-day washout period followed by bid dosing. Part B was a dose-escalation study in patients with FGFR-amplified tumours with an RP2D of 80 mg bid. *Cohort 4 dose was based on PK modelling data and was consistent with the latest tolerated exposures from AZD4547 bid dosing in Western patients [16], as well as emerging safety data from Japanese patients (this study); †In schedule 2, it was planned that dose assessment could extend over multiple cohorts; however, no cohorts exceeded the 160 mg qd dosing level due to emerging data from the study in Western patients and a decision from the clinical project team. RP2D, recommended Phase II dose