Figure 1. Del-1–deficiency increases pathological ischemia-induced angiogenesis.
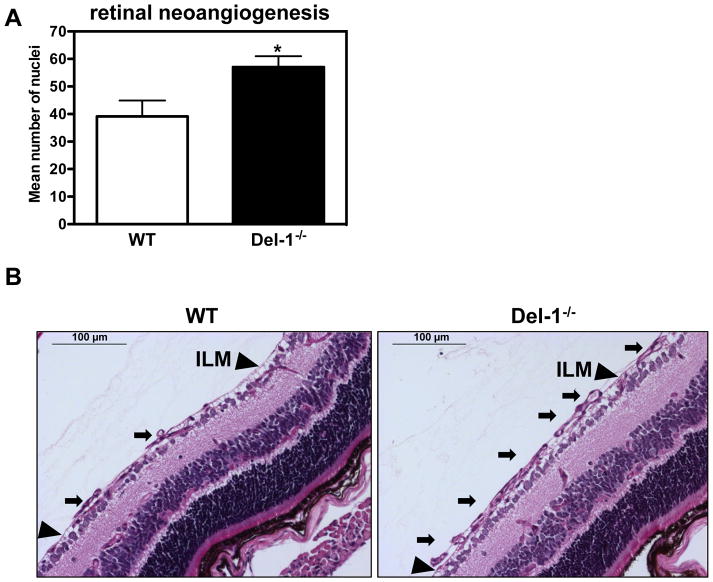
(A) Quantification of neovascularization in the ROP model was performed by counting the epiretinal neovascular nuclei of vessels anterior to the inner limiting membrane (ILM) (on the vitreal side of ILM) in PAS stained retinal cross-sections of Del-1–deficient (Del-1−/−) and wild type (WT) mice on P17. Data are mean ± SEM (*P <0.05 vs. WT mice; n=11–22). (B) Representative images of epiretinal neovascular tufts (on the vitreal side of ILM) in PAS stained retinal cross-sections; scale bar=100 μm. The black arrows indicate pathological neovascular tufts; the black arrowheads indicate the ILM.
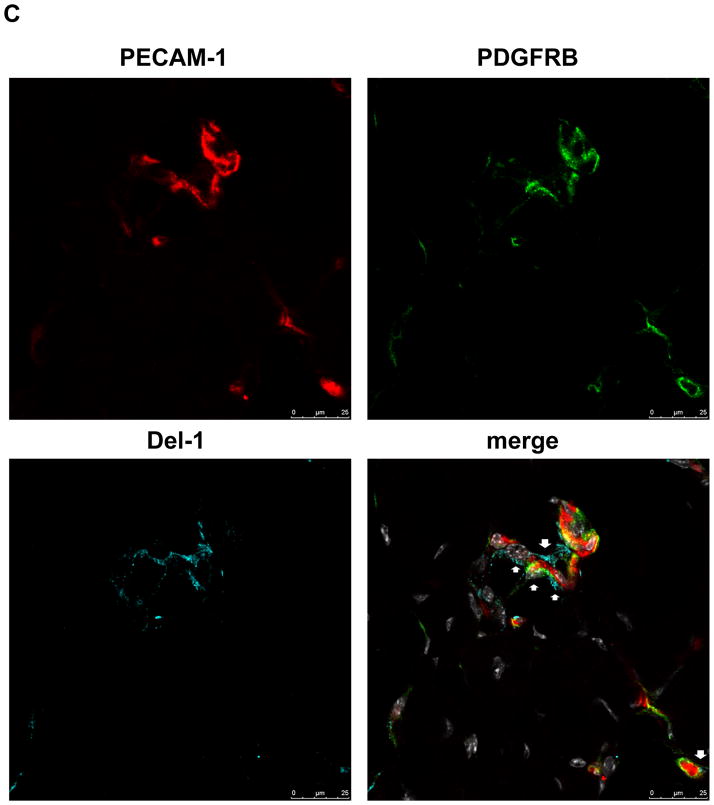
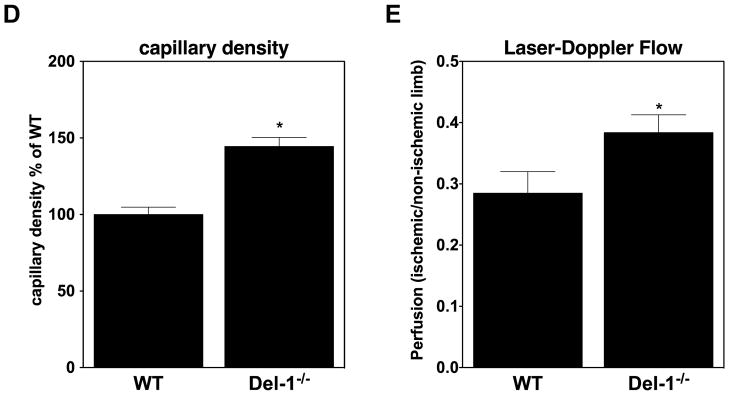
(C) Immunofluorescence staining in ischemic muscles for Del-1 (light blue fluorescence), PECAM1 (endothelial cell marker, red fluorescence), PDFGRB (pericyte and SMC marker, green fluorescence). White color indicates DAPI nuclear staining. The white arrows indicate colocalization of Del-1 with endothelial cells and pericytes/SMC and also Del-1 localization in perivascular space. (D and E) Hind limb ischemia was performed in WT and Del-1−/− mice. After 14 days, perfusion (E) of ischemic limbs was determined by laser Doppler imaging. The ischemic muscles were harvested and the capillary density (D) was assessed microscopically as described in Materials and Methods. (*P <0.05 vs. WT mice; n=8).