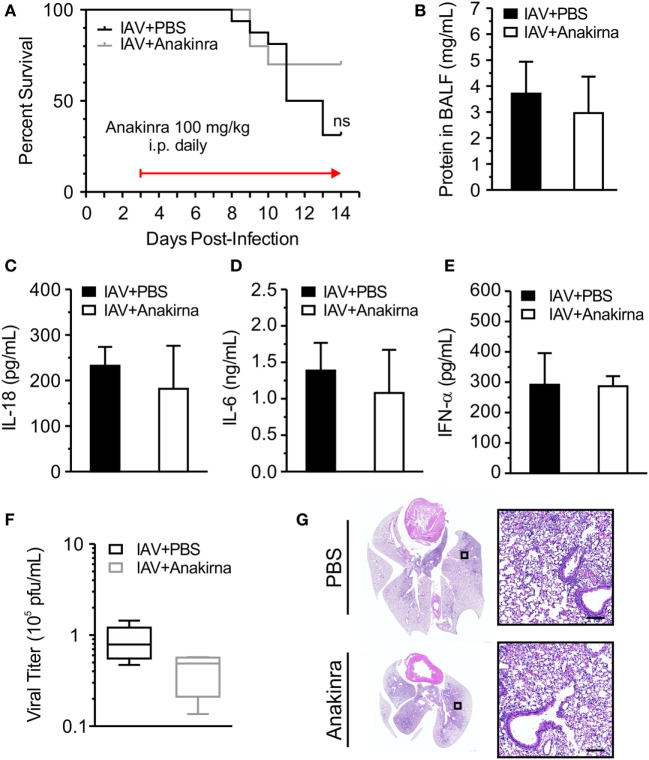
Figure 6.
Anakinra treatment does not protect juvenile mice from influenza A virus (IAV) infection. Juvenile mice were infected with IAV (WSN 12.5 plaque forming unit intratracheal) and treated with anakinra [100 mg/kg intraperitoneal (i.p.) daily] or vehicle control beginning on day 3 postinfection (p.i.). (A) Mortality. (B) Protein in bronchoalveolar lavage fluid (BALF) on day 7 p.i. (C–E) Interleukin-18 (IL-18), interleukin-6 (IL-6), and interferon-α (IFN-α) in BALF on day 7 p.i. as measured by ELISA. (F) Viral titer on day 7 p.i. was measured by plaque assay. (G) Hematoxylin and eosin stained lung sections from juvenile mice 7 days p.i. with IAV and treatment with anakinra or phosphate-buffered saline (PBS) control. Images shown are representative of three mice for each condition. Scale bars, 100 µm.