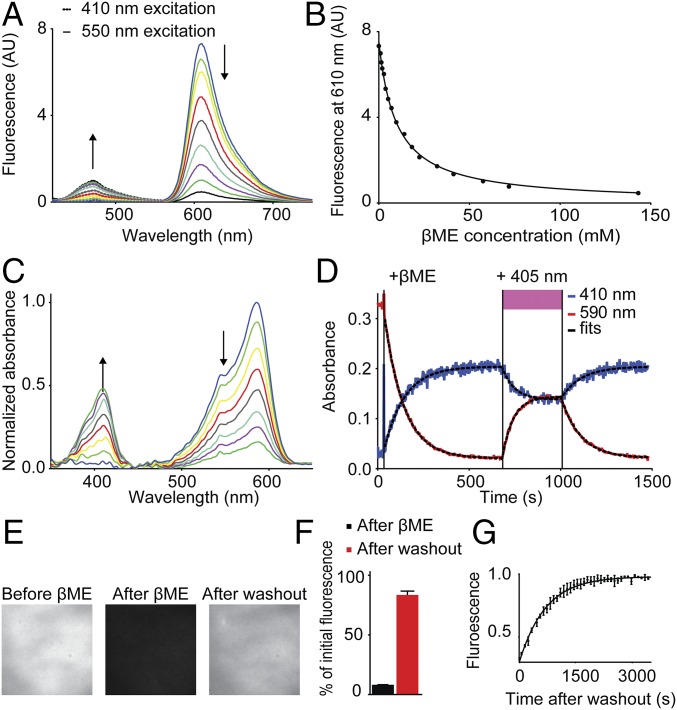
Fig. 3.
βME transforms mCherry into a blue fluorescent protein. (A) Fluorescence spectra of in vitro mCherry molecules incubated with various βME concentrations, excited with 410 nm light (dotted lines) and 550 nm light (solid lines). Arrows indicate increasing βME concentration. (B) Quantification of mCherry fluorescence intensity at 610 nm as function of βME concentration. Solid line represents a fit of the fluorescence intensity according to an equation for a reversible bimolecular reaction I = I0 /(1 + Keq⋅[βME]), yielding Keq = 0.10 mM−1 (N = 1, n = 4). (C) Absorbance spectra of in vitro mCherry molecules over time after addition of 286 mM βME. Arrows indicate increasing time. (D) Absorbance of purified mCherry molecules at 410 nm (black line) and 590 nm (red line) as a function of time. Addition of 286 mM βME and exposure to 405 nm light are indicated. Dotted black lines represent fits for one phase decay: I = Plateau + (I0 − Plateau)·exp(−t/τ). Values for τ are 111 s−1 and 106 s−1 after βME, 65 s−1 and 60 s−1 during 405 nm illumination, and 109 s−1 and 108 s−1 after 405 nm illumination, for absorbance at 410 nm and 590 nm, respectively. (E) Fluorescence images of purified mCherry molecules on a coverslip before addition of 286 mM βME, after addition of βME, and 1.5 h after washout of βME. (F) Quantification of the fluorescence signal after addition of 286 mM βME (8% ± 0.1%) and 1.5 h after washout (83% ± 3%) (mean ± SEM; N = 2, n = 44 regions). (G) Kinetics of fluorescence return after βME washout. Fluorescence intensity is normalized to the final value. The solid line represents a fit for one phase dissociation of βME and mCherry, yielding τ = 7.0·102 s (95% confidence interval 6.6·102–7.4·102; N = 3).