Fig. 2.
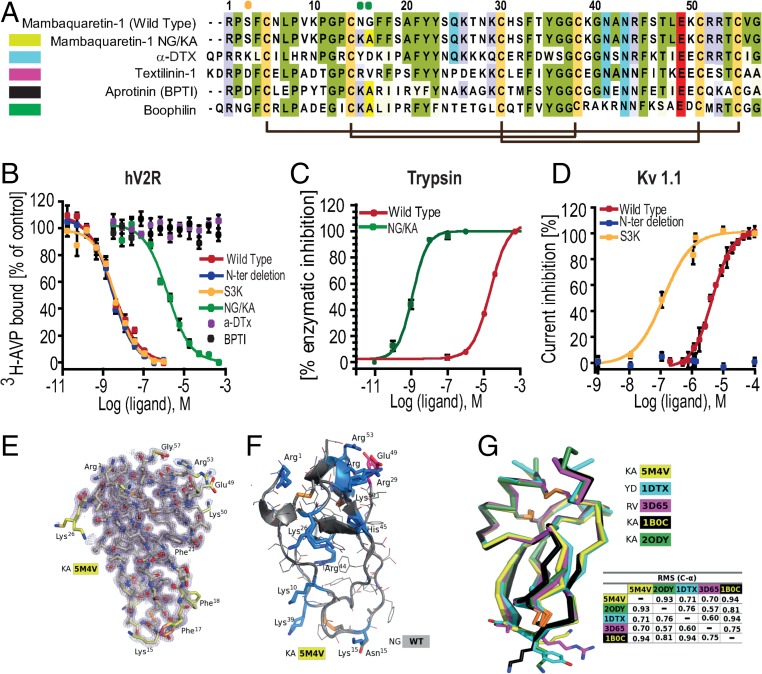
Mambaquaretin-1 binds V2R with its first loop. (A) Sequence comparison between mambaquaretin-1, its KA variant, -DTX, Australian common brown snake textilinin-1, aprotinin (BPTI), and cattle tick clotting inhibitor boophilin. Cysteines are shaded light green, positively charged residues are shaded blue, negatively charged residues are shaded red, and other conserved residues are shaded gray. Orange and green circles indicate residues critical for -DTX and BPTI activity, respectively. (B) Binding inhibition of 3H-AVP on hV2R by mambaquaretin-1, its three variants, BPTI, and -DTX. (C) Trypsin inhibition by mambaquaretin-1 and its NG/KA variant. (D) Current inhibition of Kv1.1 channels obtained by plotting the percentage of blocked current in function of increasing toxin concentrations by mambaquaretin-1, N-ter deletion, and S3K variant. (E) X-ray structure of mambaquaretin-1 KA in stick representation colored according to B value (light blue to red). (F) Cartoon representation of mambaquaretin-1 and the KA variant showing the preponderance of positively charged residues. (G) Superposition on C- of mambaquaretin-1 KA (PDB ID: 5M4V), -DTX [PDB ID: 1DTX (17)], textilin-1 [PDB ID: 3D65 (18)], BPTI [PDB ID: 1B0C (19)], and boophilin [PDB ID: 2ODY (20)] with rmsd between the structures from PyMOL alignment.