Fig. 1.
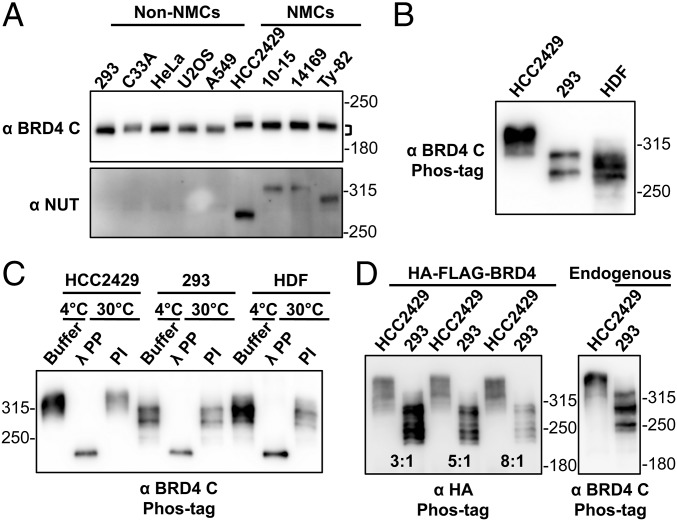
BRD4 is hyperphosphorylated in NMCs. (A) Whole-cell lysates of non–NMCs and NMCs were resolved on SDS/PAGE and immunoblotted with BRD4 C-terminal domain (BRD4 C) and NUT antibodies. Brackets mark the difference in the position of NMC and non–NMC BRD4. (B) Whole-cell lysates of HCC2429 and HEK293 cells and HDFs were resolved on a Phos-tag gel and immunoblotted with BRD4 C antibody. Molecular weight markers are shown on the right. However, as indicated by the manufacturer, the molecular weight markers are frequently distorted during Phos-tag gel electrophoresis, and therefore they can be used only as rough estimates of the molecular weights. (C) BRD4 immunoprecipitated from HCC2429 and HEK293 cells and HDFs was mixed with buffer, λPP, or PIs. Samples were incubated at 4 °C or 30 °C as indicated. Proteins then were analyzed on Phos-tag gel and immunoblotted with BRD4 C antibody. (D) HCC2429 and HEK293 cells were transfected with a construct expressing HA-FLAG–tagged BRD4. At 2 d posttransfection, whole-cell lysates were analyzed on Phos-tag gel and immunoblotted with HA antibody. Because of the different transfection efficiencies of the two cell lines, the lysates of HCC2429 and HEK293 were loaded at the ratios of 3:1, 5:1, and 8:1 as indicated. Lysates from untransfected HCC2429 and HEK293 cells were also loaded on the same Phos-tag gel to show the endogenous BRD4 controls. The samples were immunoblotted with BRD4 C antibody. See also Fig. S1.