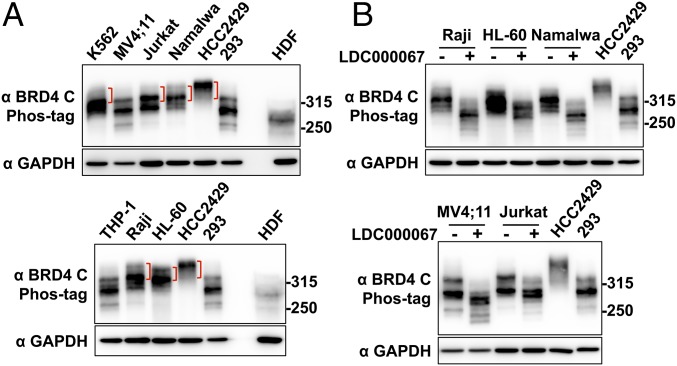
Fig. 6.
BRD4 is also hyperphosphorylated in non–NMC cancer cells associated with BRD4 dysfunction. (A) Whole-cell lysates from various leukemia and lymphoma cells were analyzed on a Phos-tag gel and compared with the samples from HCC2429 and HEK293 cells and HDFs. The samples were immunoblotted with BRD4 C antibody. Brackets mark the hyperphosphorylated BRD4 bands. The samples were also analyzed on SDS/PAGE and immunoblotted with GAPDH. Because the BRD4 protein level in HDFs is extremely low, seven times more HDF protein sample was loaded in the Phos-tag gel than in the SDS/PAGE for the GAPDH blot. The images shown are representative of three independent experiments. (B) Leukemia and lymphoma cells were treated with DMSO or 10 μM LDC000067 for 2 h. Whole-cell lysates were analyzed on Phos-tag or SDS/PAGE gel and were immunoblotted with the indicated antibodies. Lysates from untreated HCC2429 and HEK293 cells were also loaded on the same Phos-tag gel to show the endogenous BRD4. See also Fig. S6.