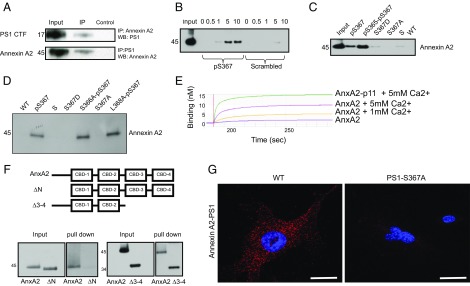
Fig. 2.
PS1 phosphorylated at Ser367 binds Annexin A2. (A) Immunoprecipitates (IP) from whole-mouse brain lysate using anti-Annexin A2 or anti-PS1 antibody were immunoblotted with an antibody against PS1 or Annexin A2. WB, Western blot. (B) Pull-down, showing that increasing the concentration of calcium increases the binding between recombinant Annexin A2 and the PS1-pSer367 biotinylated peptide. Numbers indicate calcium concentration (millimolar). P, phosphorylated PS1 peptide; S, phosphorylated scrambled control peptide. (C) Recombinant Annexin A2 binds to both PS1-pSer367 biotinylated peptide and a double-phosphorylated PS1-pSer365-pSer367 biotinylated peptide. It does not bind to S367D or S367A biotinylated peptide or to an S or WT sequence biotinylated peptide. (D) Recombinant Annexin A2 binds to PS1-pSer367 biotinylated peptide and to a mutant S366A-pS367 and L368A-pS367 biotinylated peptide. It does not bind to a S367D or S367A biotinylated peptide or to an S or WT sequence biotinylated peptide. (E) Biolayer interferometry between a PS1-pSer367 biotinylated peptide and an Annexin A2 (AnxA2) or AnxA2-p11 fusion protein at several Ca2+ concentrations. The y axis represents binding (nanometers). (F, Upper) Diagram showing the constructs used in this experiment. (F, Lower) Deletion mutant of Annexin A2 lacking the N terminus (ΔN) does not bind a pPS1 peptide, whereas a deletion mutant of Annexin A2 lacking the third and fourth calcium-binding domains (Δ3–4) is still able to bind a pPS1 peptide. (G) In situ PLA between Annexin A2 and PS1 in MEFs derived from WT or PS1-S367A mice. Note the loss of Annexin A2-PS1 binding in the absence of PS1 phosphorylation at Ser367. (Scale bars, 2 μm.)