Abstract
Background
Pharmacokinetic data for lopinavir in late pregnancy and in breastfeeding are limited, and no data for abacavir in breast milk are available.
Methods
Women in the Mma Bana Study initiated HAART from 18 to 34 weeks of gestation. We determined trough plasma and whole breast milk concentrations of lopinavir (LPV), abacavir (ABC), nevirapine (NVP), lamivudine (3TC) and zidovudine (ZDV) among separate subsets of pregnant and breastfeeding women, and in plasma of exposed infants. Lopinavir was measured 1 month after starting HAART or 1 month postpartum, and other drugs were measured 1 month postpartum.
Results
Sampling occurred a median of 14 h (range 11–17) from last maternal drug ingestion. Although 50% higher median LPV levels were seen in postpartum than antepartum plasma (8.29 μg/ml versus 5.51 μg/ml; P=0.02), antepartum levels with standard LPV dosing were therapeutic for all women (>1.0 μg/ml). Very low LPV levels (<0.25 μg/ml) were detected in breast milk. Median ABC levels in breast milk were 85% of those in plasma (0.057 μg/ml versus 0.067 μg/ml). Breast milk concentrations of NVP and 3TC were 27% and 74% of plasma levels, respectively. At these trough maternal time points, only NVP was detectable in potentially inhibitory levels in breastfeeding infants, and most infants had non-detectable levels of LPV, ABC, ZDV and 3TC via maternal breast milk.
Conclusions
Standard LPV dosing achieved therapeutic levels in pregnancy and no appreciable concentrations in breast milk. ABC is detectable in breast milk at similar concentrations to plasma, but does not result in appreciable infant exposure.
Introduction
The use of HAART for the prevention of mother-to-child transmission (PMTCT) of HIV is expanding globally. Recent studies suggest that maternal HAART during breastfeeding is an effective strategy for late PMTCT in areas of the world where avoidance of breastfeeding is not safe or feasible [1–4], but little is known regarding antiretroviral (ARV) levels in breast milk or in the blood of breastfeeding infants [5–8]. Levels of abacavir (ABC) have not been previously described in human breast milk, and there are limited data for lopinavir (LPV). In addition, lower levels of LPV have been described in the third trimester of pregnancy compared with postpartum values in some studies [9], but the clinical implication of this finding is not known.
We previously reported results for the Mma Bana Study in Botswana [1], where HIV-infected women received one of three possible ARV regimens during pregnancy and through 6 months of breastfeeding. This pharmacological sub-study was designed to determine plasma and breast milk concentrations of LPV, ABC, nevirapine (NVP), lamivudine (3TC) and zidovudine (ZDV) among breastfeeding women, and in the plasma of their infants, at a known time after maternal drug ingestion. We also evaluated plasma LPV concentrations in women during the third trimester of pregnancy compared with the postpartum period.
Methods
Study population
We studied a subset of women and their infants enrolled in an ongoing randomized clinical trial to prevent mother-to-child transmission at four sites in Botswana. Known as the Mma Bana Study (meaning ‘mother of the baby’ in Setswana), this study began enrolment in July 2006 and completed enrolment of 730 HIV-infected women in May 2008. Women with CD4+ T-cell counts ≥200 were randomized to receive either ABC (300 mg)/ZDV (300 mg)/3TC (150 mg) co-formulated as Trizivir (GlaxoSmithKline, Greenford, UK) twice daily (nucleoside reverse transcriptase inhibitor [NRTI]-arm) or LPV (400 mg)/ritonavir (100 mg; LPV/r) with ZDV (300 mg)/3TC (150 mg) co-formulated as Kaletra (Abbott, Abbott Park, IL, USA)/Combivir (GlaxoSmithKline) twice daily (protease inhibitor-arm). Women with CD4+ T-cell counts <200 cells/mm3 or with AIDS-defining illness received what was then standard-of-care treatment for Botswana: NVP (200 mg)/ZDV (300 mg)/3TC (150 mg) twice daily (following a 2-week lead-in period of 200 mg NVP once daily, plus ZDV [300 mg]/3TC [150 mg] twice daily [observational arm]). Randomized women initiated HAART between 26 and 34 weeks of gestation and continued through weaning or 6 months postpartum (whichever came first), and obser vational women initiated HAART between 18 and 34 weeks of gestation and continued indefinitely. All infants received a single 6 mg dose of NVP at birth, and ZDV at 4 mg/kg twice daily for 4 weeks. Maternal breast milk was collected at 1 month postpartum among breastfeeding women.
Sampling protocol
All blood samples in this protocol were taken just prior to subsequent dose, representing a trough concentration. In total, 20 women provided plasma samples 1 month after initiating HAART in pregnancy for LPV sampling; 10 were receiving the original LPV/r capsule formulation, and 10 were receiving the newer LPV/r tablet formulation (after July 2007). A total of 15 women receiving LPV (6 of whom were also sampled in pregnancy, and all but 1 receiving the tablet formulation) provided plasma and breast milk samples at 1 month postpartum, and their infants provided a plasma sample for LPV/3TC/ZDV testing. For women receiving ABC/3TC/ZDV or NVP/3TC/ZDV, all pharmacokinetic testing was postpartum. At 1 month, 15 women in each group provided plasma and breast milk, and their infants provided plasma, for testing of all ARVs. In addition, 15 infants (5 from each treatment group) had plasma drawn for ZDV testing at 3 months of age to avoid sampling infants while receiving direct prophylaxis.
Study procedure
The Health Research Development Committee from Botswana and the Harvard School of Public Health Human Subjects Committee approved the study protocol and amendments, and informed consent was provided by all mothers in the study. Prior to pharmacological sampling, a questionnaire was completed to record maternal ARV dosing history (date and time of last three doses), the time of last infant feeding and the time of last infant prophylactic ZDV dose. In total, 5 ml of whole breast milk and 1 ml of infant blood were obtained as part of routine Mma Bana follow-up or for the purposes of this study. An additional 3 ml of maternal blood was drawn for the purposes of this study. The time of collection was documented for all specimens. Maternal demographic data were collected as part of the Mma Bana Study.
All samples were stored in EDTA tubes at −4°C for a maximum of 8 h before being transported to the Botswana-Harvard HIV Reference Laboratory on the day of collection. At the laboratory, plasma and breast milk samples were frozen at −70°C prior to shipment.
Drug assays from blood and breast milk
All samples were sent to the Pediatric Pharmacology Research Laboratory at the University of California, San Diego for pharmacokinetic analysis.
Plasma
Infant and maternal plasma ZDV concentrations were measured by a validated enzyme immunoassay (EIA; available commercially from Neogen Corporation, Lansing, MI, USA). The calibration range for the assay was 5–1,250 ng/ml. Assay sensitivity was 10 ng/ml. Intra- and inter-day precisions ranged from 7.4 to 13.6%. Coefficient of variation (CV) and accuracy ranged from −8.6 to 9.9% deviation. Infant plasma 3TC and NVP concentrations were determined by a validated liquid chromatography-mass spectrometry (LC-MS) assay because of small volumes. Assay sensitivity was 7 ng/ml for 3TC and 16 ng/ml for ABC and NVP. Maternal plasma 3TC, ABC and NVP concentrations were measured by validated reversed phase high-pressure liquid chromatography assay using UV detection. Assay sensitivity was 32 ng/ml for 3TC, 58 ng/ml for ABC and 43 ng/ml for NVP.
Breast milk
The percentage of fat in each breast milk sample was roughly estimated by taking a known volume of well-mixed whole milk (usually 1 ml) and weighing in a pre-weighted tube. After centrifugation for 5–10 min at 14,000×g, the milk under the fat layer was carefully removed and kept for assay (called skimmed milk). The tube was re-weighed and the percentage of fat estimated by dividing the weight of the fat by the weight of the whole milk ×100.
Whole milk was assayed for all drugs using LC-MS methods. The 3TC infant plasma assay described above was used to measure 3TC in breast milk. Assay sensitivity was 34 ng/ml. Precision within the assay ranged from 3 to 13.5% CV, and accuracy ranged from 0.8 to 8% deviation. Milk sample ZDV, LPV, ABC and NVP concentrations were measured simultaneously using a validated LC-MS assay. Assay sensitivity was 35 ng/ml for LPV, ABC and NVP and 45 ng/ml for ZDV. The intra- and inter-day precisions were 1.1–10% CV for ZDV, 1.6–9% CV for ABC, 1.2–12% CV for NVP and 0.7–8.9% CV for LPV. Intra- and inter-day accuracy ranged from −8.9 to 4% deviation for ZDV, −4.9 to 11.6% for ABC, −3.1 to 10.5% for NVP and −0.5 to 7.7% for LPV. In vitro experimentation demonstrated that the recoveries of drug (within the analytical ranges of each assay) for each drug in skimmed versus whole milk were as follows: 3TC (72 versus 94.7%), ZDV (103.5 versus 93.4%), NVP and LPV (889.2 versus 90.7%) and ABC (84 versus 95.3%).
The UCSD Pediatric Pharmacology Research Laboratory participates in a rigorous proficiency testing (PT) programme twice a year through its role within the ACTG Clinical Pharmacology Quality Assurance Program. PT plasma samples were assayed for NVP, ABC, LPV, ZDV and 3TC during the plasma assays described above and passed the acceptance criteria for the programme.
Statistical analyses
Statistical testing was performed using SAS V9.1 (SAS Institute Inc., Cary, NC). The medians of ARV concentration parameters and their approximate 95% CIs were obtained, excluding samples known to be outside a 10–18 h window from last dose. Pearson correlation coefficients were used to evaluate the relationship between milk to maternal plasma concentration ratios and time from maternal dosing to sample collection. The Kruskal–Wallis test was used for comparisons of two groups.
Results
We enrolled 50 women and their infants in this pharmacokinetic substudy. The median length of time that women had received continuous HAART before antenatal sampling was 28 days (range 27–30), and sampling occurred at a median of 32 weeks gestation (range 30–39). The median length of time that women had received continuous HAART before postpartum sampling was 105 days (range 53–182), and sampling occurred at a median of 30 days postpartum (range 28–33). The median age for women participating in this study was 27 years (range 19–46), the median weight at antenatal sampling was 65 kg (range 52–103), and at 1 month postpartum it was 62 kg (range 43–96). The median pretreatment CD4+ T-cell count was 348 cells/mm3 (range 47–1,342 cells/mm3).
Sufficient maternal sample was available for 49 LPV tests (20 antenatal plasma, 15 postnatal plasma, 14 breast milk), 30 ABC tests (15 postnatal plasma, 15 breast milk), 30 NVP tests (15 postnatal plasma, 15 breast milk), 22 ZDV tests (10 postnatal plasma, 12 breast milk) and 59 3TC tests (14 plasma, 45 breast milk). Sufficient infant plasma was available for 5 LPV tests, 9 ABC tests, 9 NVP tests, 33 ZDV tests (24 at 1 month, 9 at 3 months) and 24 3TC tests.
A total of 4 women with last confirmed ARV ingestion outside a window of 10–18 h were excluded from the analysis. The median time between ingestion of the last dose of ARVs and maternal sampling was 14 h (range 11–17 h) for those with complete dosing information. All participants reported adherence with their three most recent ARV doses. All infants were breastfeeding (either exclusively or mixed feeding), and the median time between their most recent breastfeeding and blood sampling was 1 h (range 6 min–35 h).
The median maternal plasma and breast milk LPV, ABC, NVP, 3TC and ZDV trough concentrations, and the median breast milk to plasma ratio for each, are shown in Table 1.
Table 1.
ARV concentrations in maternal plasma and breast milka
| ARV | Median maternal plasma concentration during third trimester of pregnancy, μg/ml | Median maternal plasma concentration at 1 month postpartum, μg/ml | Median maternal breast milk concentration at 1 month postpartum, μg/ml | Median maternal breast milk to plasma ratio at 1 month postpartumb |
|---|---|---|---|---|
| LPVc | Capsule: 6.18 | Capsule: 7.12 | 0.06 | 0.007 |
| Tablet: 4.85 | Tablet: 10.75 | |||
| Total: 5.51 | Total: 8.29 | |||
| ABCd | – | 0.067 | 0.057 | 0.85 |
| NVP | – | 6.71e | 1.83e | 0.27 |
| 3TC | – | 0.19e | 0.14e | 0.74 |
| ZDV | – | – | 0.007e | – |
Half-maximal inhibitory concentration (IC50) for lopinavir (LPV)=0.0019 μg/ml, IC50 for abacavir (ABC)=0.07 μg/ml, IC50 for nevirapine (NVP)=0.024 μg/ml, IC50 for lamivudine (3TC)=0.55 μg/ml, IC50 for zidovudine (ZDV)=0.005 μg/ml [10,15].
Median of individual breast milk to plasma ratios for each participant.
Results exclude women with peak below detectable limit (two antepartum plasma samples, eight postpartum milk samples).
Results exclude women with peak below detectable limit (six plasma samples, five milk samples).
Results exclude women with detectable peaks sampled outside of a 10–18 h range from last dose (four breast milk 3TC, one plasma 3TC; one breast milk NVP, one plasma NVP; two breast milk ZDV). ARV, antiretroviral.
The maternal ZDV plasma samples likely encountered hydrolysis (from ZDV-glucuronide) and are not reported. Although 50% higher median LPV levels were seen postpartum than antepartum (P=0.02), antepartum levels with standard LPV dosing were in the therapeutic range for wild-type virus (>1.0 μg/ml) in all women. No significant differences were detected between women who received capsule formulation and women who received tablet formulation. Very low LPV levels were detected in breast milk. Median ABC levels in breast milk were 85% of the plasma level and detectable for most women, despite the expected low trough concentrations for a nucleoside analogue. Figure 1 shows the drug concentrations in plasma and breast milk for each woman by time between drug ingestion and sample collection.
Figure 1. Plasma and breast milk concentration of LPV, NVP, ABC and 3TC, by hours between dose and sample collection, at the antepartum or postpartum collection.
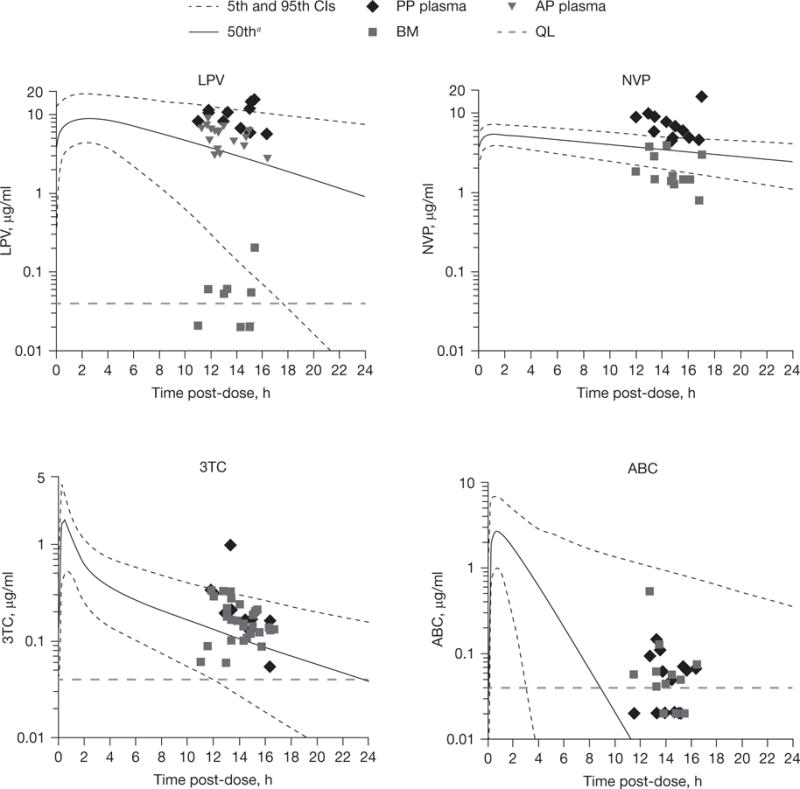
aMedian and theoretical curves. Median and 95% CI curves are theoretical curves based on published parameters for plasma. ABC, abacavir; AP, antepartum; BM, breast milk; LPV, lopinavir; NVP, nevirapine; PP, postpartum; QL, quantitation limit; 3TC, lamivudine.
Infant exposure through breastfeeding was determined for all drugs and measured at 1 month (or 3 months for ZDV, after discontinuation of direct ZDV prophylaxis at the 1 month visit). Few infant specimens yielded detectable drug concentrations for either LPV (2/5; both <1 μg/ml), ABC (1/9) or 3TC (0/23). Several infants receiving ZDV prophylaxis had significant ZDV concentrations at 1 month. However, at the 3 month visit (after infant ZDV prophylaxis had been discontinued for all infants), no infants had detectable ZDV concentrations (0/9). All 9 infants with available samples whose mothers were receiving NVP had detectable concentrations in their blood, with a median level of 0.37 μg/ml (range 0.24–1.2 μg/ml), which is >100× the half-maximal inhibitory concentration-(0.0024–0.024 μg/ml) [10]. The median infant plasma NVP concentration was approximately 6% of the median maternal value.
Discussion
We detected therapeutic trough levels of LPV in the third trimester of pregnancy, and higher levels in the postpartum period. We also performed the first analysis of ABC in breast milk and detected a median level of 85% of plasma. Of the five ARVs studied, only NVP was detected at potentially inhibitory levels in breastfeeding infants.
Lower LPV levels have been reported in some (but not all) studies in late pregnancy [7,9,11,12], but the clinical implications of this finding are not clear. Several cohorts have found adequate HIV RNA suppression at delivery with Kaletra-based HAART using standard dosing (400/100 twice daily), including the parent study for this pharmacokinetic analysis [1,13,14]. In the Mma Bana Study, after a median of 11 weeks on treatment, 93% of women who initiated Kaletra-based HAART were suppressed to <400 copies/ml at delivery, and 69% were suppressed to <50 copies/ml. These values are consistent with studies among non-pregnant patients. We now demonstrate that standard Kaletra dosing achieved therapeutic trough levels in all women evaluated, which supports the use of standard dosing in pregnancy to avoid additional cost and programme complexity. By contrast to at least one previous study [15], the newer tablet formulation had a lower median trough concentration than the capsule formulation; however, this difference was not statistically significant and is unlikely to be clinically relevant.
The pharmacokinetics of ABC in breast milk appear similar to 3TC and other NRTIs, with the possible exception of ZDV. ABC enters breast milk and is present at a concentration slightly below plasma [16]. Although this finding suggests a possible benefit for reducing breast milk HIV RNA concentrations, it also raises the concern for the development of de novo resistance mutations to ABC in breast milk and the possibility of transmitting these to a breastfeeding infant, as with other NRTIs. In this study, ZDV was detected at low trough concentrations in breast milk. This is consistent with at least one other study [6], but contrasts with another [5], and the possibility of hydrolysis from ZDV-glucuronide (as likely occurred with maternal plasma) cannot be entirely excluded. LPV demonstrated decreased penetration into breast milk compared with other ARVs in this study. High protein binding in plasma may limit LPVs partitioning into breast milk, which contains casein as its primary protein. Although LPV is relatively lipophillic, the breast milk fat (3–5%) is apparently insufficient to result in high concentrations of LPV in whole breast milk.
Only NVP was detectable in infants at potentially inhibitory concentrations, which is consistent with previous studies [5,6]. For each of the other ARVs tested, a minority of infants had detectable levels in the sub-therapeutic range. These low-but-detectable levels are of concern for breastfeeding infants who become HIV infected, but the benefit of HAART for preventing infection is likely to exceed this risk.
Limitations of this study included the availability of only pre-dose (trough) concentrations and the lack of peak values or repeated values for each ARV, as well as the need to exclude samples from four women that were outside a reasonable trough window. The time from last dose to sampling was also missing for four trough samples. The sample size was small for LPV, ABC and NVP, but similar to other studies [6,7,8,9].
In summary, we provide reassuring data that LPV concentrations in the third trimester were therapeutic for all women tested, and the first evidence that ABC passes into breast milk. The results may inform PMTCT programme decisions and help to predict resistance patterns among HIV-infected infants.
Acknowledgments
We are indebted to the patients who participated in the Mma Bana Study, and thank the individuals who helped with the study (see Additional file 1).
This study was supported by a grant from the National Institute of Allergy and Infectious Diseases, National Institutes of Health (U01-AI066454); by GlaxoSmithKline (which provided Trizivir and Combivir); by Abbott Pharmaceuticals (which provided Kaletra); by the government of Botswana (which provided Nevirapine, Zidovudine and Lamivudine); and by NICHD PPRU Network grants at the University of California, San Diego 1U10 HD045937-01.
The study sponsors had no role in study design, in the collection, analysis and interpretation of data, in the writing of the report or in the decision to submit this paper for publication.
Footnotes
Disclosure statement
EC has received <$5,000 in consulting fees for Abbott Laboratories. The remaining authors declare no competing interests.
Additional file
Additional file 1: A list of individuals who helped with the study can be found at http://www.intmedpress.com/uploads/documents/AVT-12-OA-2741_Shapiro_Add_file1.pdf
References
- 1.Shapiro RL, Hughes MD, Ogwu A, et al. Antiretroviral regimens in pregnancy and breast-feeding in Botswana. N Engl J Med. 2010;362:2282–2294. doi: 10.1056/NEJMoa0907736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thomas T, Masaba R, Ndivo R, et al. Prevention of mother-to-child transmission of HIV-1 among breastfeeding mothers using HAART: The Kisumu Breastfeeding Study, Kisumu, Kenya, 2003–2007. 15th CROI; 3–6 February 2008; Boston, MA, USA. Abstract 45aLB. [Google Scholar]
- 3.Kesho Bora Study Group. de Vincenzi I. Triple antiretroviral compared with zidovudine and single-dose nevirapine prophylaxis during pregnancy and breastfeeding for prevention of mother-to-child transmission of HIV-1 (Kesho Bora study): a randomised controlled trial. Lancet Infect Dis. 2011;11:171–180. doi: 10.1016/S1473-3099(10)70288-7. [DOI] [PubMed] [Google Scholar]
- 4.Chasela CS, Hudgens MG, Jamieson DJ, et al. Maternal or infant antiretroviral drugs to reduce HIV-1 transmission. N Engl J Med. 2010;362:2271–2281. doi: 10.1056/NEJMoa0911486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shapiro RL, Holland DT, Capparelli E, et al. Antiretroviral concentrations in breast-feeding infants of women in Botswana receiving antiretroviral treatment. J Infect Dis. 2005;192:720–727. doi: 10.1086/432483. [DOI] [PubMed] [Google Scholar]
- 6.Mirochnick M, Thomas T, Capparelli E, et al. Antiretroviral concentrations in breast-feeding infants of mothers receiving highly active antiretroviral therapy. Antimicrob Agents Chemother. 2009;53:1170–1176. doi: 10.1128/AAC.01117-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mirochnick M, Best BM, Stek AM, et al. Lopinavir exposure with an increased dose during pregnancy. J Acquir Immune Defic Syndr. 2008;49:485–491. doi: 10.1097/QAI.0b013e318186edd0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mirochnick M, Fenton T, Gagnier P, et al. Pharmacokinetics of nevirapine in human immunodeficiency virus type 1-infected pregnant women and their neonates. Pediatric AIDS Clinical Trials Group Protocol 250 Team. J Infect Dis. 1998;178:368–374. doi: 10.1086/515641. [DOI] [PubMed] [Google Scholar]
- 9.Stek AM, Mirochnick M, Capparelli E, et al. Reduced lopinavir exposure during pregnancy. AIDS. 2006;20:1931–1939. doi: 10.1097/01.aids.0000247114.43714.90. [DOI] [PubMed] [Google Scholar]
- 10.Parkin NT, Hellmann NS, Whitcomb JM, Kiss L, Chappey C, Petropoulos CJ. Natural variation of drug susceptibility in wild-type human immunodeficiency virus type 1. Antimicrob Agents Chemother. 2004;48:437–443. doi: 10.1128/AAC.48.2.437-443.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramautarsing RA, van der Lugt J, Gorowara M, et al. Thai HIV-1-infected women do not require a dose increase of lopinavir/ritonavir during the third trimester of pregnancy. AIDS. 2011;25:1299–1303. doi: 10.1097/QAD.0b013e328347f7e9. [DOI] [PubMed] [Google Scholar]
- 12.Cressey TR, Jourdain G, Rawangban B, et al. PHPT-5 Team Pharmacokinetics and virologic response of zidovudine/lopinavir/ritonavir initiated during the third trimester of pregnancy. AIDS. 2010;24:2193–2200. doi: 10.1097/QAD.0b013e32833ce57d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lyons F, Lechelt M, De Ruiter A. Steady-state lopinavir levels in third trimester of pregnancy. AIDS. 2007;21:1053–1054. doi: 10.1097/QAD.0b013e3281053a1e. [DOI] [PubMed] [Google Scholar]
- 14.Bouillon-Pichault M, Jullien V, Azria E, et al. Population analysis of the pregnancy-related modifications in lopinavir pharmacokinetics and their possible consequences for dose adjustment. J Antimicrob Chemother. 2009;63:1223–1232. doi: 10.1093/jac/dkp123. [DOI] [PubMed] [Google Scholar]
- 15.Else LJ, Douglas M, Dickinson L, Back DJ, Khoo SH, Taylor GP. Improved oral bioavailability of lopinavir in melt-extruded tablet formulation reduces impact of third trimester on lopinavir plasma concentrations. Antimicrob Agents Chemother. 2012;56:816–824. doi: 10.1128/AAC.05186-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumar PN, Sweet DE, McDowell JA, et al. Safety and pharmacokinetics of abacavir (1592U89) following oral administration of escalating single doses in human immunodeficiency virus type 1-infected adults. Antimicrob Agents Chemother. 1999;43:603–608. doi: 10.1128/aac.43.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]


