Abstract
Intracellular membrane fusion steps in eukaryotes require the syntaxin family of SNARE proteins. Syntaxins are regulated at several levels through interactions with regulatory proteins, including the Sec1p/Munc18 (SM) proteins. Key to understanding this regulation is the characterisation of different SM-syntaxin binding interactions, both at the molecular level and their contribution to function in vivo. The most conserved SM-syntaxin binding mode is through interaction of the syntaxin’s extreme N-terminal peptide with a hydrophobic pocket on the surface of the SM protein. Surprisingly, mutant versions of two different SM proteins abrogated for this binding display no discernable phenotypes in vivo. In this issue of the Biochemical Journal, Johnson et al. demonstrate that loss of the N-terminal binding interaction between the syntaxin UNC-64 and the SM protein UNC-18 severely impairs neuromuscular synaptic transmission in Caenorhabditis elegans, resulting in an uncoordinated phenotype. In contrast, loss of a second mode of SM-syntaxin binding has no detectable effect. Collectively, these data suggest that, although different membrane trafficking steps are all regulated by SM-syntaxin interactions using similar binding modes, they are differentially regulated, highlighting the need for careful dissection of the binding modes.
Keywords: Sec1p/Munc18 (SM), syntaxin, SNARE, membrane fusion
Different SM-Syntaxin Binding Modes
One of the defining features of eukaryotic cells is their compartmentalisation into physically and functionally distinct membrane-bound organelles. Eukaryotic cells maintain their intracellular architecture by using transport vesicles to deliver protein and lipid cargo between organelles. It is imperative that membrane fusion events are tightly regulated, both spatially and temporally, as failure to do so would have disastrous consequences for the cell. Central to the process of membrane fusion are members of the SNARE (soluble N-ethylmaleimide-sensitive factor attachment protein receptor) protein family. Formation of trans SNARE complexes between SNAREs in opposing lipid bilayers is required to drive membrane fusion; thus, regulating SNARE complex assembly provides the cell with a means of regulating membrane fusion. Sec1p/Munc18 (SM) proteins are essential for SNARE-mediated membrane fusion and, like the SNAREs, are conserved throughout evolution. Understanding the conserved roles of these proteins has proved challenging, largely due to the divergent modes of binding detected between SM proteins and their cognate SNAREs.
Functional SNARE complexes are composed of four SNARE motifs, at least one of which is contributed by a syntaxin protein. In addition to the coiled-coil SNARE motif, syntaxins possess an autonomously folded N-terminal Habc domain. For many syntaxins, such as the mammalian neuronal syntaxin1a (Sx1a), this Habc domain binds intramolecularly to the SNARE motif region; this closed conformation is inhibitory to SNARE complex formation. The crystal structure of the Munc18a-Sx1a complex revealed that the SM protein is an arch-shaped molecule that cradles syntaxin in its closed conformation (Mode 1; Figure 1A; [1, 2]). This discovery led to the hypothesis that SM proteins inhibit membrane fusion by stabilising the closed conformation of syntaxin (for review, see [3]).
Figure 1. Binding interactions between SM and syntaxin proteins.
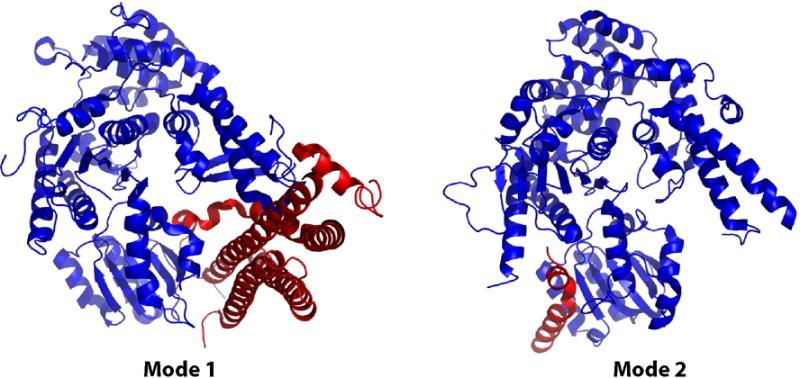
Mode 1 (left): The closed conformation of the mammalian neuronal syntaxin1a (red) binds in the cleft of the SM protein Munc18a (blue; Burkhardt; PDB ID 3c98). Mode 2 (right): The N-terminal peptide of yeast Sed5p binds in a hydrophobic pocket on the surface of the SM protein Sly1p (Bracher; PDB ID 1mqs). Similar N-peptide binding is observed in the Munc18c-syntaxin4 structure (Hu; PDB ID 2pjx) and in this re-refined Munc18a-syntaxin1a (not shown).
However, other members of the SM protein family were found to employ a strikingly different mechanism to bind their cognate syntaxins, as observed in the Sly1p-Sed5p crystal structure (Figure 1B; [4]). As expected from its homology with Munc18a (21% sequence identity), Sly1p has a similar structure. However, rather than the closed conformation of Sed5p binding in the inner cleft of the Sly1p arch, the extreme N-terminal peptide of Sed5p (residues 1–21) binds to a hydrophobic pocket on the outer face of Sly1p. This second mode of binding (Mode 2) is compatible with binding syntaxin either in a closed conformation, or in an open conformation. As such, Mode 2 binding is compatible with models in which the SM does not inhibit SNARE complex assembly, but rather the SM can remain bound to the assembled SNARE complex, to facilitate downstream events (for review, see [3]). Although indirect perturbation of Mode 2 binding in mammalian cells appears to result in trafficking defects, its direct abolition in two different SM proteins in the yeast Saccharomyces cerevisiae, Sly1p and Vps45p, yields no discernable phenotype ([5, 6], and references therein).
This lack of a phenotype for disruption of Mode 2 binding has been puzzling, because Mode 2 is more conserved than Mode 1. Syntaxin N-terminal peptide binding has been demonstrated biochemically and structurally for several other SM proteins, including Munc18c [7] and the endosomal SM Vps45p [5]. Moreover, recent evidence suggests that several SM-syntaxin pairs can use more than one mode of binding, e.g. Munc18a can bind to Sx1a using both Modes 1 and 2 [1]. In addition, residues outside of the N-peptide region of mammalian Syntaxin 16 increase its affinity for Vps45, suggesting a second binding mode [1]. The challenge now is to dissect the functions of these different modes of binding in vivo.
The Syntaxin N-peptide is Required for Neurotransmission
To investigate the functional relevance of the different SM-syntaxin modes of binding, Johnson et al. created mutant versions of the C. elegans Munc18a homologue, UNC-18, predicted to selectively disrupt either Mode 1 or Mode 2 binding to the Sx1a homolgue UNC-64 [8]. Indeed, these mutations impair binding to UNC-64 in vitro. The authors then investigated the physiological consequences of disrupting these modes of binding in vivo. A version of UNC-18 whose Mode 1 binding to UNC-64 is disrupted (R39C) rescues the thrashing and locomotion defects of unc-18 mutant worms in a manner indistinguishable from the wild-type protein. In contrast, a version impaired in Mode 2 binding (F113R) is unable to do so. These data indicate that Mode 1 binding of UNC-18 to syntaxin is dispensable for function, whereas Mode 2 binding is essential for neurotransmission. Concurrent with this study, McEwen et al. found similar neuromuscular trafficking defects using a mutation in the UNC-64 N-terminal peptide (L9A) that blocked Mode 2 binding [9]. These studies are an important first demonstration of a functional requirement for Mode 2 binding between an SM protein and its cognate syntaxin in vivo and nicely mirror studies performed using an in vitro liposome fusion assay to examine the consequences of disrupting interactions between Sx1a and Munc18a [10].
These intriguing results, however, are difficult to reconcile with previous studies in yeast that found no requirement for Mode 2 binding between the SM proteins Sly1p and Vps45p and their cognate syntaxins Tlg2p and Sed5p, respectively [5, 6]. How can this discrepancy be resolved? It is possible that Mode 2 binding is only required for specialized exocytic processes, such as release of neurotransmitter, whereas Sed5p-Sly1p and Tlg2p-Vps45p both regulate constitutive transport steps. However, the sequences required for Mode 2 binding are conserved across syntaxin-SM pairs required for different trafficking steps in diverse eukaryotes, suggesting involvement in a fundamental process. Perhaps for SMs such as yeast Sly1p and Vps45p, other binding interactions are compensatory upon loss of Mode 2 binding, but have not been uncovered yet. Mammalian Vps45 binds Sx16 through Mode 2, but additional interactions, suggestive of Mode 1 binding have recently been revealed by quantitative binding experiments [1]. The answer may also be that the in vivo functional assays used in the Sly1p and Vps45p yeast studies are not sensitive enough to reveal a disruption of Mode 2 function. For example, the L117R mutation in yeast Vps45p, which abrogates Mode 2 binding, has no obvious phenotype by itself; however, in combination with the dominant negative W244R mutation, the dominant negative phenotype is eliminated. This suggests that N-terminal peptide binding does have a role in Vps45p function [5].
SM proteins have been implicated in many different functions in the SNARE complex assembly/disassembly cycle: as a chaperone to facilitate trafficking of the cognate syntaxin to the correct membrane; as an inhibitor to keep the syntaxin closed to ensure spatial and temporal specificity of SNARE complex assembly; as a regulator, in conjunction with other factors, to release the closed conformation of the syntaxin and stimulate SNARE assembly; and as a stimulator of membrane fusion (for review, see [3]). Although each SM protein might regulate its cognate SNARE cycle by these mechanisms, it is likely that the rate-limiting step in each SNARE assembly/disassembly cycle varies for different trafficking steps. Consequently, disrupting the mode of SM-syntaxin interaction that regulates a non-rate-limiting stage in the cycle may not result in an obvious phenotype. Thus, the process regulated by Mode 2 binding of UNC18 to UNC64 may be rate-limiting for the fusion of neurotransmitter vesicles, but the trafficking pathways regulated by Vps45p and Sly1p in yeast may have a different rate-limiting step (not regulated by Mode 2 binding).
Progress is clearly being made towards understanding the functions of the different modes of SM-syntaxin binding, and specific non-interacting mutants, such as those employed by Johnson et al., represent powerful tools to facilitate this process. Similarly, specific in vitro functional assays, such as SNARE complex assembly and liposome fusion assays, are making progress toward teasing out the separate functions [1, 10]. An important aspect missing from most SM-syntaxin studies has been quantitative evaluation of the relative affinities of these binding sites. Qualitative pull-down binding studies and yeast two-hybrid assays have clearly added to our understanding of SM-syntaxin interactions, but they do not accurately report the binding affinities of the different mutant proteins. This has recently been demonstrated by an isothermal titration calorimetry study of Sx1a-Munc18a and Sx16-Vps45 [1]. This study indicates that Munc18a binds tightly to the closed Sx1a, and weakly to its N-peptide, whereas Vps45 binds tightly to the syntaxin16 N-peptide and very weakly to the closed syntaxin16. Because the relative affinities for Mode 1 vs. Mode 2 in these two mammalian SM proteins are very different, these results may not be reliable predictors for other SM homologues. Indeed, in the study by Johnson et al., abrogation of either Mode 1 or Mode 2 binding of UNC-18 to UNC-64 severely inhibits the interaction assessed by pull-down experiments, suggesting that both the UNC-64 N-peptide and closed conformation contribute to the overall affinity for UNC-18, rather than Mode 1 being dominant as in the case of the mammalian counterparts (Munc18a/Sx1a). This highlights the need to take care when making extrapolations between different SM-syntaxin pairs and underscores the need for quantitative binding studies. These types of quantitative studies, combined with specific point mutations and in vivo assays, as used by Johnson et al., is an important goal of future research, and will contribute greatly to our understanding of the functions of SM proteins in SNARE-mediated membrane fusion.
Contributor Information
Nia J. Bryant, Email: n.bryant@bio.gla.ac.uk.
Mary Munson, Email: mary.munson@umassmed.edu.
References
- 1.Burkhardt P, Hattendorf DA, Weis WI, Fasshauer D. Munc18a controls SNARE assembly through its interaction with the syntaxin N-peptide. EMBO J. 2008;27:923–933. doi: 10.1038/emboj.2008.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Misura KM, Scheller RH, Weis WI. Three-dimensional structure of the neuronal-Sec1-syntaxin 1a complex. Nature. 2000;404:355–362. doi: 10.1038/35006120. [DOI] [PubMed] [Google Scholar]
- 3.Toonen RF, Verhage M. Vesicle trafficking: pleasure and pain from SM genes. Trends Cell Biol. 2003;13:177–186. doi: 10.1016/s0962-8924(03)00031-x. [DOI] [PubMed] [Google Scholar]
- 4.Bracher A, Weissenhorn W. Structural basis for the Golgi membrane recruitment of Sly1p by Sed5p. EMBO J. 2002;21:6114–6124. doi: 10.1093/emboj/cdf608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carpp LN, Ciufo LF, Shanks SG, Boyd A, Bryant NJ. The Sec1p/Munc18 protein Vps45p binds its cognate SNARE proteins via two distinct modes. J Cell Biol. 2006;173:927–936. doi: 10.1083/jcb.200512024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peng R, Gallwitz D. Multiple SNARE interactions of an SM protein: Sed5p/Sly1p binding is dispensable for transport. EMBO J. 2004;23:3939–3949. doi: 10.1038/sj.emboj.7600410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hu SH, Latham CF, Gee CL, James DE, Martin JL. Structure of the Munc18c/Syntaxin4 N-peptide complex defines universal features of the N-peptide binding mode of Sec1/Munc18 proteins. Proc Natl Acad Sci USA. 2007;104:8773–8778. doi: 10.1073/pnas.0701124104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson JR, Ferdek P, Lian LY, Barclay JW, Burgoyne RD, Morgan A. Binding of UNC-18 to the N-terminus of syntaxin is essential for neurotransmission in Caenorhabditis elegans. Biochem J. 2008 doi: 10.1042/BJ20081956. [DOI] [PubMed] [Google Scholar]
- 9.McEwen JM, Kaplan JM. UNC-18 promotes both the anterograde trafficking and synaptic function of syntaxin. Mol Biol Cell. 2008;19:3836–3846. doi: 10.1091/mbc.E08-02-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shen J, Tareste DC, Paumet F, Rothman JE, Melia TJ. Selective activation of cognate SNAREpins by Sec1/Munc18 proteins. Cell. 2007;128:183–195. doi: 10.1016/j.cell.2006.12.016. [DOI] [PubMed] [Google Scholar]


