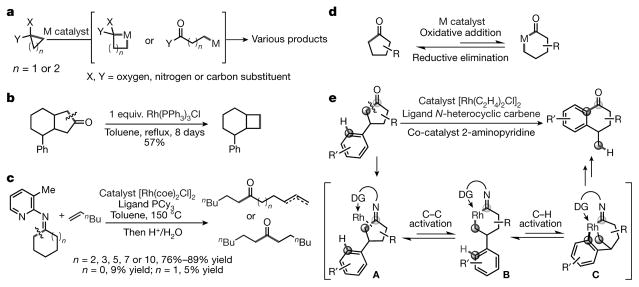
Figure 1. Activation of C–C bonds in ring systems.
a, Catalytic C–C activation of strained rings (for example, in cyclopropane or cyclobutanone). The unfavourable energetics of C–C activation can be compensated by the release of strain in the ring. M, transition metal. b, Stoichiometric rhodium-mediated C–C activation of less strained cycloketones. This reaction is less efficient than that shown in a. Ph, phenyl. c, Directed catalytic C–C activation of less strained cycloketimines. The yield is high for large cycloketimines, but low for smaller ones. Bu, butyl; coe, cyclooctene; Me, methyl; PCy3, tricyclohexylphosphine. d, The challenge in terms of activating the C–C bonds of cyclopentanones is that C–C activation is reversible; the thermodynamic driving forces do not always allow oxidative addition with a transition metal, instead favouring the reverse process (C–C reductive elimination). R, hydrocarbon side chain. e, Our strategy for catalytic C–C activation of cyclopentanones: merging the unfavourable C–C activation with C–H activation, to produce an overall thermodynamically favoured reaction. Specifically, when using a cyclopentanone with an aryl group in the C3 position, the imine intermediate (A; with a pendant directing group, DG) should allow the transient C–C activation intermediate (B) to undergo an intramolecular C–H activation, producing rhodacycle (C). Subsequent reductive elimination will lead to α-tetralone derivatives.