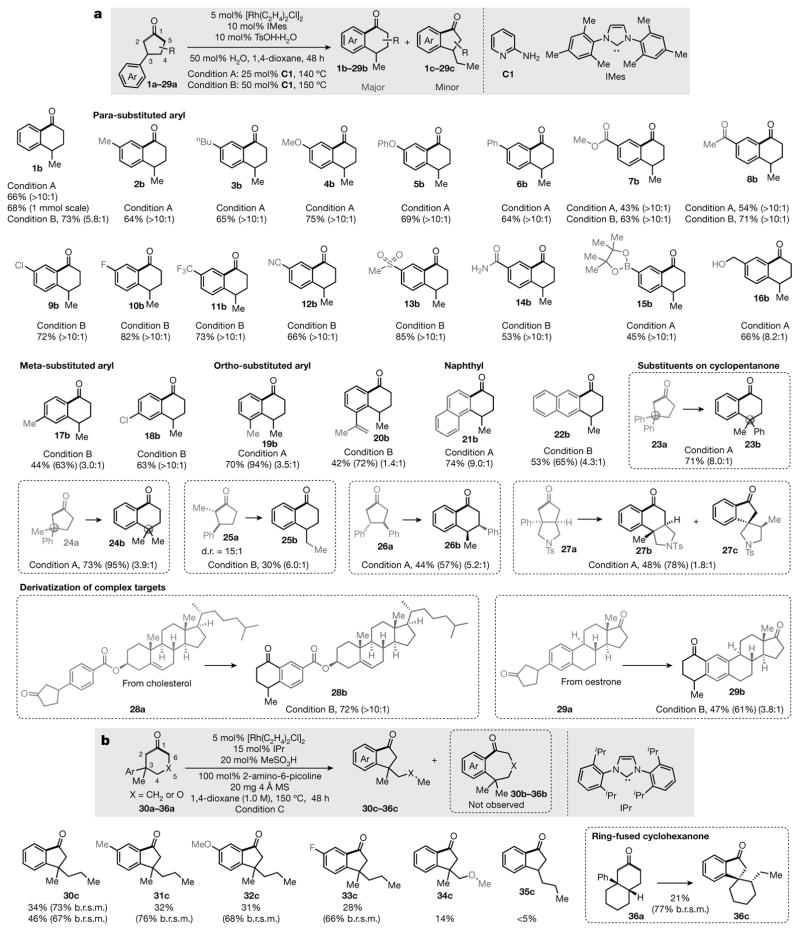
Figure 2. Substrate scope.
a, Scope of the cyclopentanones. The shaded box at the top shows the basic reaction: the substrates are 3-arylcyclopentanones (1a–29a); the major products, α-tetralones (1b–29b), come from cleavage of the more hindered C1–C2 bond, while the minor products, α-indanones (1c–29c), are generated through cleavage of the less hindered C1–C5 bond. [Rh(C2H4)2Cl]2 is the catalyst precursor and 2-aminopyridine (C1) is the co-catalyst; TsOH is toluene sulfonic acid. Ar, aryl group. Below the shaded box are shown the major products and their isolated yields. b, Scope of the cyclohexanones. See Supplementary Information for further experimental details. The regioselective ratios (r.r.) were determined by gas chromatography-mass spectrometry or 1H nuclear magnetic resonance (NMR) of the crude products. The percentages in parentheses are the total yields or the b.r.s.m. (based on recovered starting material) yields determined by 1H NMR, using 1,1,2,2-tetrachloroethane (TCE) as the internal standard. d.r., diastereomeric ratio; IMes, 1,3-bis(2,4,6-trimethylphenyl)imidazol-2-ylidene; IPr, 1,3-bis(2,6-diisopropylphenyl)imidazol-2-ylidene; MS, molecular sieve.