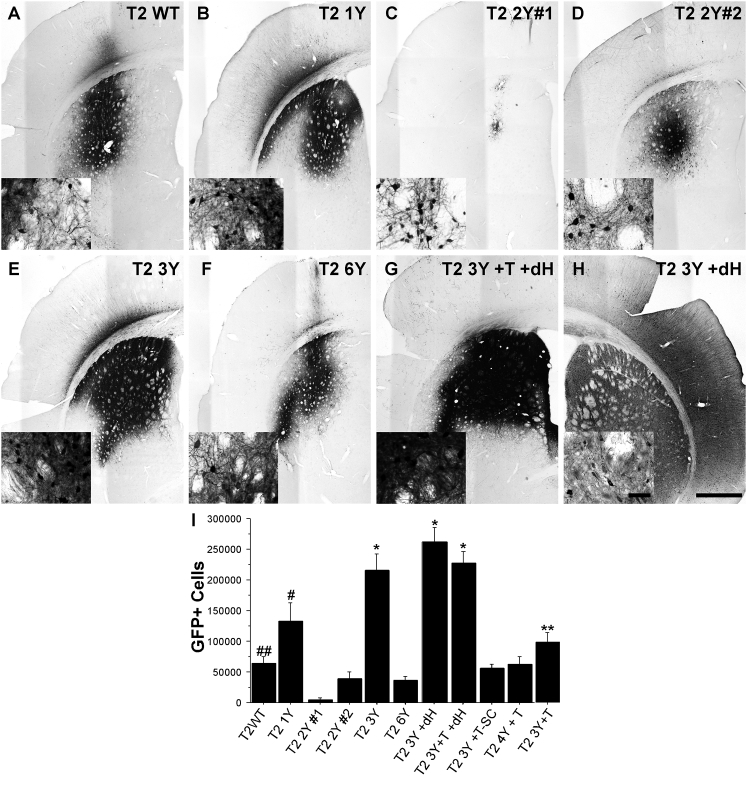
Figure 1.
Rationally Designed AAV2 Capsid Mutants Significantly Enhance Striatal Transduction
Adult Sprague-Dawley rats received intrastriatal injections of an AAV2 vector (2 μL of 1.2 × 1012 vg/μL for all viruses) as defined in Table 1. One month later, the animals were sacrificed and processed for transgene (GFP) immunoreactivity. (A–H) Representative images of GFP immunoreactivity in the striatum following the injection of (A) T2 WT (n = 8), (B) T2 1Y (n = 7), (C) T2 2Y#1 (n = 6), (D) T2 2Y#2 (n = 8), (E) T2 3Y (n = 7), (F) T2 6Y (n = 8), (G) T2 3Y +T +dH (n = 12), and (H) T2 3Y +dH. Insets are higher magnification images taken at the periphery of the transduction area. (I) Stereological quantification of striatal GFP+ neurons revealed that T2 3Y, T2 3Y +T +dH, and T2 3Y +dH transduced significantly more striatal neurons than any other capsid (*p < 0.001) followed by T2 1Y (#p < 0.004 versus remaining groups except T2 3Y +T). Several capsid serotypes did not exhibit an improvement over WT (T2 2Y#2, T2 6Y, T2 4Y +T, T2 3Y +T), and T2 2Y#1 exhibited significant impairment in transduction as compared with the T2 WT control (##p < 0.03). The T2 3Y +T capsid showed higher transduction versus T2 2Y#1, T2 2Y#2, and T2 6Y (**p < 0.01). (I) Error bars represent mean + SD. (H) Scale bar, 1 mm (inset scale bar, 50 μm); scale bar applies to all micrographs.