Figure 2.
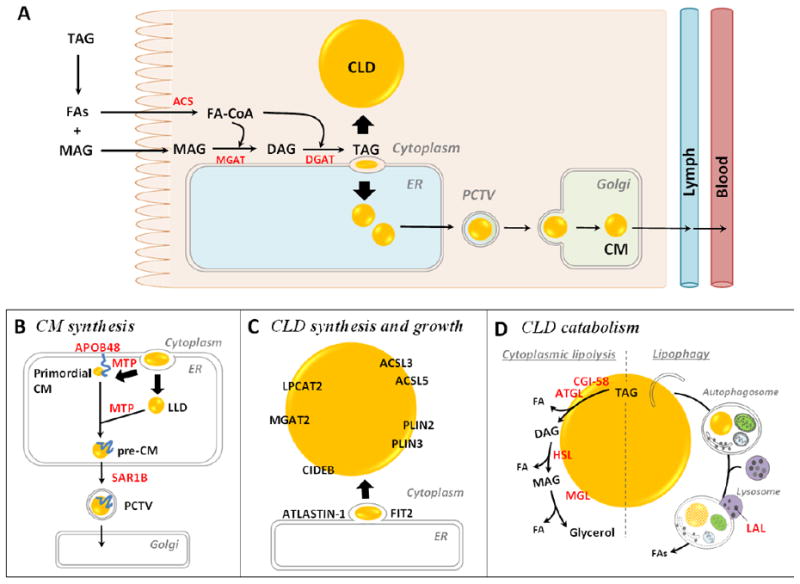
(A) Molecular mechanisms of dietary fat absorption within enterocytes. Triacylglycerol (TAG) is hydrolyzed in the lumen of the gastrointestinal tract by lipases, generating fatty acids (FAs) and 2- monoacylglycerol (MAG). These digestive products are taken up by enterocytes. The FAs are primarily activated to fatty acyl-CoAs by acyl-CoA synthetase (ACS) activity and utilized for TAG re-synthesis through the acyl-CoA:monoacylglycerol acyltransferase (MGAT) pathway. The first step involves the synthesis of diacylglycerol (DAG) from 2-MAG and a fatty acyl-CoA by MGAT activity. The second, final step involves the synthesis of TAG from DAG and a fatty acyl-CoA by acyl-CoA:diacylglycerol acyltransferase (DGAT) activity. The synthesized TAG within ER membrane bilayer is trafficked towards either the cytoplasm for cytoplasmic lipid droplet (CLD) synthesis or the ER lumen for chylomicron (CM synthesis). (B) Mechanism and proteins involved in CM synthesis. CMs are made by a two-step pathway within the ER. The first step is the synthesis of primordial CMs, as neutral lipids are packaged on apolipoprotein B48 (ApoB48). The second step is the expansion of the CM core by adding neutral lipids from ApoB-free lumenal lipid droplets (LLDs). The activity of microsomal triglyceride transfer protein (MTP) is essential for the synthesis of primordial CMs and for CM growth in the ER lumen. Pre-CMs exit the ER and are transported to the Golgi for further processing via a prechylomicron transport vesicle (PCTV) containing a coat protein complex II (COPII). SAR1B, one of the core components of COPII complex, initiates PCTV budding from ER and directs PCTV to Golgi. CMs are eventually released from the basolateral side of the enterocyte by Golgi-derived secretory vesicles. (C) Mechanisms and proteins involved in enterocyte CLD synthesis and growth. The most accepted model of CLD synthesis is budding of excess TAG from the smooth ER membrane bilayer with specific proteins involved in the process. Multiple proteins are identified on CLDs and shown to mediate local CLD growth. Lysophosphatidylcholine acyltransferase 2 (LPCAT2) synthesizes phosphatidylcholine, MGAT2 synthesizes DAG, and ACSLs synthesize fatty acyl-CoAs, providing lipids substrates for CLD growth. PLINs stabilize CLD structure. Cell death inducing DFF45-like effector B (CIDEB), fat storage-inducing transmembrane protein 2 (FIT2) and ATLASTIN-1 are also shown to regulate CLD size. (D) Mechanisms and proteins involved in enterocyte CLD catabolism. Cytoplasmic lipolysis and lipophagy are two identified mechanisms of CLD catabolism. The lipases contributing to these two pathways are present in enterocytes. In cytoplasmic lipolysis, adipocyte triglyceride lipase (ATGL) (with its co-activator comparative gene identification-58 (CGI-58)), hormone sensitive lipase (HSL), and MAG lipase (MGL) sequentially cleave FAs from the glycerol backbone of TAG by hydrolyzing ester bonds. In lipophagy, CLDs are selectively targeted and sequestered within autophagosomes and then transported to the lysosome where they are hydrolyzed by lysosome acidic lipase (LAL). The FAs released from CLDs in enterocytes may serve as substrates for re-esterification of TAG, PL, and CE for secretion on lipoproteins or membrane synthesis, fatty acid oxidation (FAO), or serve roles as signaling molecules.
