Abstract
Aims
Increasing evidence suggests that levels of pro-inflammatory and anti-inflammatory cytokines are dysfunctional in alcohol dependence. Moreover, some initial findings demonstrate that these adaptations in peripheral inflammation may contribute to motivation for alcohol and problem drinking via possible direct effects or the indirect effects of stress responsivity. Importantly, the role of pro-inflammatory and anti-inflammatory cytokines in the progression from healthy to problem drinking is not well understood. The aim of the current study was to assess whether alcohol-related peripheral immune system changes affect stress and alcohol cue-induced craving and anxiety and behavioral alcohol motivation and intake in the laboratory among problem drinkers compared with socially drinking controls.
Methods
Twenty-six problem drinkers and 38 moderate, social drinkers participated in a laboratory challenge procedure during which they were exposed to 3 personalized 5-minute imagery conditions (stress (S), relaxing (R) and alcohol cue (C), followed by the “alcohol taste test” (ATT) as a measure of implicit alcohol motivation and intake, presented across 3 consecutive days, one per day in a randomized and counterbalanced order. Measures of Tumor Necrosis Factor alpha (TNFα), Interleukin-6 (IL-6), Interleukin-1 receptor antagonist (IL-1ra), alcohol craving and anxiety were assessed at baseline, immediately following imagery exposure and at discreet beer cue presentation in the ATT.
Results
Compared with moderate drinkers, problem drinkers demonstrated tonic attenuation of IL-6 and IL-1ra. In problem drinkers, these changes also accompanied elevated levels of stress and cue-induced alcohol craving, anxiety, and were predictive of provoked alcohol craving, behavioral alcohol motivation and intake and severity of problem drinking.
Conclusions
Current findings indicate that selective immunosuppression in problem drinkers may play a key role in motivation for alcohol intake.
Keywords: immune system, cytokines, stress, problem drinking, alcohol intake
Graphical Abstract
This study assessed immune system changes in problem drinkers compared with socially drinking controls and whether these changes affect alcohol related behaviors. Problem drinkers compared with social drinkers demonstrated tonic attenuation of IL-6 and IL-1ra. These changes accompanied elevated levels of stress and cue-induced alcohol craving, anxiety, and predicted provoked alcohol craving, behavioral alcohol motivation and intake and severity of problem drinking. These findings indicate that immunosuppression in problem drinkers may play a key role in motivation for alcohol intake.
INTRODUCTION
Problem drinking is considered a risk factor for the development of alcohol dependence (Halme et al., 2008). Understanding some of the mechanisms that underlie the progression from social to problem drinking is therefore of importance, as it may allow for the identification of potential intermediary check stops on the way to alcohol dependence. This may aid in the development of novel and effective treatment strategies.
Increasing evidence suggests that levels of pro-inflammatory and anti-inflammatory cytokines are distorted in alcohol dependence. For example, peripheral immune system inflammation has been associated with chronic consumption of alcohol as well as alcohol dependence in individuals with and without liver disease (Christensen and Kessing, 2001, Laso et al., 2007, Laso et al., 1999). Moreover, a polymorphism in the IL-10 cytokine gene has been found to be more prevalent in alcoholics, suggesting that cytokine gene expression may be associated with the risk for development of alcohol dependence (Marcos et al., 2008). In light of findings from both clinical and preclinical research in the last decade, there is evidence to support several processes through which peripheral cytokines may impinge upon drinking behaviors in humans.
First, adaptations within selective peripheral cytokine markers may be linked to the etiology of negative affective mood states that are similar to those associated with the negative reinforcing effects of alcohol. For example, pathological activation of inflammatory mediators such as TNFα and IL-6 are associated with depressive symptoms in chronically ill patients (Maes et al., 2009), and treatment of patients with pro-inflammatory cytokines (Dunn et al., 2005) can produce similar deleterious moods to those associated with negative reinforcement for alcohol during withdrawal (Litt and Cooney, 1999, Fox et al., 2007). In terms of a potential mechanism, more recent studies have indicated that artificially-induced peripheral inflammation modulates extracellular norepinephrine and amygdaloid pro-inflammatory mRNA levels, serving to increase hypothalamic CRF, depressive symptoms and negative affect (Bienkowski and Rinaman, 2011, Engler et al., 2011, Serrats and Sawchenko, 2006).
Second, changes in peripheral immune system cytokines may also underpin the ability to regulate emotionally and cognitively in the face of stress. For example, studies have shown that enhanced systemic inflammation caused by bacterial endotoxin has potent modulatory effects on neural circuits and processes robustly associated with both affective processing and regulation, including insula, cingulate, and amygdala (Hannestad et al., 2012b, Harrison et al., 2009). Endotoxin-induced changes in glucose metabolism have also been documented within insula and subgenual anterior cingulate regions (Hannestad et al., 2012b, Harrison et al., 2009) associated with impulse control as well as regulatory control of emotion, interoception, and depressive symptoms (Craig, 2003, Inagaki et al., 2012, Baune et al., 2010).
Notably, extensive clinical and experimental data exist indicating that both negative affect and ability to regulate in the face of stress and challenge are particularly pertinent to the development of addictive processes (Sinha, 2001). This may be due to the fact that stress and negative mood are pivotal in depleting psychological resources required for effective emotional control and impinge upon behaviors important in addiction outcomes, such as impulse control, organizational skills and decision making (Blume and Marlatt, 2009).
While recent evidence suggests a prominent role for immune system adaptations in early abstinent alcoholics (Fox et al., 2010b), very little is known about whether peripheral cytokines are altered in non-dependent “at risk” drinkers and whether they may play a role in alcohol craving and intake. In order to examine this, we extended our well validated laboratory paradigm used to model stress-related craving (Sinha et al., 2003, 2007, 2009; Fox et al., 2005, 2007, 2010a) by incorporating the well-established alcohol taste test (ATT), based on previous work by Marlatt and colleagues, (Caudill and Marlatt, 1975), and further adapted in our stress and cue paradigm to assess alcohol motivation and intake (Sinha, 2013; Blaine et al., 2016). Changes in plasma levels of pro-inflammatory (TNFα, IL-6) and anti-inflammatory (IL-1ra) cytokines were examined, as these markers are known to readily cross the blood brain barrier (Pan et al., 2011, Banks, 2005), are strongly linked with mood related disorders (Eyre et al., 2016, Hayley, 2011, Abbott et al., 2015), and are known to exert changes in neural circuitry associated with the development and regulation of mood (Harrison et al., 2009, Hannestad et al., 2012a). All participants were recruited from a community sample of non-dependent drinkers to better clarify the immune-endocrine and psychological mechanisms underlying problem drinking.
METHODS
Participants
Twenty-six problem drinkers and 38 moderate non-smoking social drinkers participated in the current study. The study sample was part of a larger study assessing alcohol craving and behavioral alcohol motivation among moderate and binge/heavy alcohol users (Sinha, 2013; Blaine et al., 2016). All participants were recruited from the New Haven area via advertisements placed on-line or in local newspapers, community centers and local radio. Eligibility was ascertained via an initial phone screen. All participants had to be between the ages of 18 and 45 years and able to read and write in English to at least a 6th grade level and meet stringent health requirements assessed by a specialist research nurse. All participants were required to meet criteria for moderate drinking as a minimum requirement. This was defined as not exceeding 7 standard drinks per week for women and 14 per week for men in the last year (NIAAA, 2005). A binge pattern was also acceptable (5 or more drinks in men; 4 or more drinks in women). While current or past alcohol abuse was acceptable, all participants were excluded if they met current dependence criteria for alcohol, or if they met current criteria for any psychiatric disorders. They were also excluded if they met current abuse criteria for any other substance, including nicotine, prescription opiates, methadone or medications for other acute or chronic psychiatric and / or medical conditions. Such recruitment criteria allowed for the subsequent assessment of regular, non-dependent drinkers with the recommended levels of hazardous and non-hazardous drinking (Babor et al., 2001) (see Subjective Assessments Section).
All participants gave both written and verbal informed consent and the study was approved by the Human Investigation Committee of the Yale University School of Medicine.
Screening and Intake Procedures
Potential subjects completed an initial screening over the telephone to determine eligibility based on inclusion/exclusion criteria. Following initial eligibility screening, participants were scheduled for two, 2-hour assessment and evaluation sessions conducted at the Yale Stress Center at 2 Church Street South. During the first session participants completed consent forms as well as medical, substance abuse and psychiatric health assessments including the Alcohol Use Disorders Identification Test (AUDIT; Babor et al., 2001), the Cornell Medical Index and the Structured Clinical Interview for the Diagnostic and Statistical Manual (SCID) (First et al., 1995). The Time-Line Follow-Back Interview Method (Sobell et al., 1988) was used to assess recent amounts and frequency of alcohol consumed and pattern of binge drinking in the previous 90 days, and to assess other illicit drugs and nicotine use in subjects. During the second intake session, participants completed baseline demographic and drug use questionnaires. Following this, participants received a physical examination with a research nurse specialist assessing cardiovascular, renal, hepatic, pancreatic, hematopoietic and thyroid function, in order to ensure all participants were in good health. During both appointments, subjects underwent breath alcohol testing and urine toxicology screens in order to confirm self-report of alcohol and drug information.
Following intake sessions, participants were admitted for a three day, two night stay at the Yale Center for Clinical Investigation (YCCI) located at the Yale New Haven Hospital, as part of a larger 3-day laboratory study assessing stress system function and alcohol motivation and intake in a range of social drinkers. On the day prior to the laboratory challenge studies, participants received both imagery and relaxation training and were familiarized with all laboratory procedures. Laboratory challenge procedures were then conducted across three consecutive afternoons. During these sessions, all participants were exposed to 3 personalized 5-minute imagery conditions (stress (S), neutral (N) and alcohol cue (C)), presented across 3 consecutive days, one per day in a randomized and counterbalanced order (see below for detailed description of laboratory challenge procedure).
Defining Moderate and Problem Drinking
Alcohol Use Disorders Identification Test (AUDIT) (Babor et al., 2001)
The AUDIT is a 10-item screening instrument designed to identify drinking behaviors and distinguish between low risk drinkers and individuals with hazardous and harmful patterns of alcohol consumption in order to provide the appropriate intervention. Decades of research has found the AUDIT to be an accurate measure of assessing risk of alcohol problems across gender, age, and cultures. It has high test-retest reliability (Daeppen et al., 2000) and internal consistency reliability (Ivis et al., 2000). Typically, scores >8 on the AUDIT have been used to indicate at-risk alcohol use (Babor et al., 2001). However, data from recent review studies that have compared AUDIT scores with defined alcohol related problems show that lower scores may represent a more appropriate cut-off for identifying both harmful and hazardous use in women (Bradley et al., 1998). As such, in the current study the AUDIT score cut-off for problem drinking was 8 for men and 7 for women. All participants recruited into the study completed the AUDIT during the first intake session, and were subsequently grouped by the AUDIT scores described above.
Script Development
Imagery script development was conducted before the laboratory challenges. Procedures are based on methods developed by Lang and colleagues (Lang et al., 1980, Lang et al., 1983) and further adapted in our previous studies (Fox et al., 2005, Sinha et al., 1992, Sinha et al., 2000, Sinha et al., 2003, Sinha et al., 2009). Briefly, the stress imagery script was based on subjects’ descriptions of a recent personal stressful event that made them so “sad, mad, or upset,” that they were not able to control themselves in the moment, and that was experienced as “most stressful.” “Most stressful” was determined by having the subjects rate their perceived stress on a 10-point Likert scale where 1 = not at all stressful and 10 = the most stress they felt recently in their life. Only situations rated as 8 or above were accepted as appropriate for script development (e.g., being fired from their job, marital conflict situation). The alcohol cue scripts were developed by having subjects identify a recent situation that included alcohol-related stimuli and resulted in subsequent alcohol use (e.g., walking by their favorite bar; watching others drink alcohol). Alcohol-related situations that were associated with negative affect or psychological distress were not allowed. A neutral non-physiologically arousing, and non-alcohol-related script was developed from the subjects’ description of a personal, relaxing situation (e.g., being at the beach; fall afternoon reading at the park). In addition to the script development, participants were also brought into the testing room, to acclimatize them to specific aspects of the study procedures including the subjective rating forms and training in relaxation and imagery procedures, as previously described in Sinha and colleagues (2003).
Laboratory Challenge Procedures
On each day, participants were brought into the testing room at 02:00PM, after eating a light lunch at noon. After settling into a sitting position in a hospital bed, a heparin-treated catheter was inserted by the research nurse in the antecubital region of the subject’s non-preferred arm. This was followed by a 40-minute adaptation period during which time subjects were asked to practice relaxation. At 02:40PM, following the adaptation period, baseline plasma samples were drawn and subjective rating scales administered, following which participants were provided with headphones and exposed to the imagery condition for that day. They were all given the following instructions: “Close your eyes and imagine the situation being described, ‘as if’ it were happening right now. Let your body and mind get completely involved in the situation, doing what you would do in the real situation”. Immediately following imagery exposure, all dependent measures were again collected. In addition, immediately following imagery and assessments, subjects were also presented with the “alcohol taste test” (ATT) on each day to experimentally assess motivation for alcohol consumption and amount of alcohol consumed after stress, alcohol cue and neutral imagery exposure as described previously (Sinha, 2013; Beech et al., 2014; Blaine et al., 2016). After the imagery period and ratings of anxiety and craving, subjects were presented with a tray of two 12-Oz beer mugs with chilled beer and a glass of water and ice. Subjects then participated in a 10 minute “taste test” in which they were asked to taste the beer in each mug to identify whether the brand and type of beer was the same or different in each mug. They were told that they could drink as much as they wished to make this determination and that they would receive $10 for each day for correct identification of whether the beers were same or different. The amount of beer consumed on each experimental day was recorded as a measure of behavioral alcohol motivation and intake.
Subjective measures of alcohol craving, anxiety, negative mood and biological stress and immune system measures (plasma Tumor Necrosis Factor alpha (TNFα), Interleukin-6 (IL-6), Interleukin-1 receptor antagonist (IL-1ra)) were collected at 3 time-points: baseline, immediately following imagery exposure and 10 minutes later after the presentation of the beer glasses (discrete alcohol cue) but prior to the onset of the taste test.
Laboratory Assessments: Immune System Markers
Plasma Cytokines
Plasma samples (4 mL) were collected on three consecutive days at three consecutive time-points: a) baseline, b) immediately following imagery exposure, and c) following exposure to a discrete alcohol cue. All plasma samples were collected in heparinized tubes that were immediately placed on ice after drawing. Plasma was subsequently separated by centrifugation at 4° C for 15 minutes at 1000 X g, then aliquoted and stored in polypropylene tubes at −80° C until the time of the assay. TNFα, IL-6 and IL-1ra concentrations were quantitatively determined by enzyme-linked immune-sorbent assays using the Quantikine ELISA Development Kit from R&D systems (Minneapolis, MN, USA). For TNFα, the assay kit had a sensitivity of 5.5 pg/mL, an assay range of 15.6 – 1,000 pg/mL, and specificity for natural and recombinant human TNFα. For IL-6, the assay kit had a sensitivity of 0.7 pg/mL, an assay range of 3.1 – 300 pg/mL, and specificity for natural and recombinant human IL-6. For IL-1ra, the assay kit had a sensitivity of 18.3 pg/mL, an assay range of 31.2 – 2,000 pg/mL, and specificity for natural and recombinant human IL-1ra. IL-10 and IL-1β levels were not measured due to the limited sensitivity of the assay for these particular markers. Assaying of all plasma cytokines was conducted at the Yale Center for Clinical Investigation (YCCI), Core Mineral Metabolism Laboratory.
Laboratory Assessments: Subjective
Alcohol Craving
Visual Analog Scale (VAS)
Participants were requested to rate the intensity of their desire to use alcohol at that moment using a 10-point visual analog scale (VAS) in which 0 = “not at all” and 10 = “extremely high.”
Mood and Anxiety
Anxiety Visual Analog Scale (VAS)
Participants were requested to rate how tense, anxious, and/or jittery they felt at that moment using a 10-point visual analog scale (VAS) in which 0 = “not at all” and 10 = “extremely high.”
Differential Emotion Scale (DES; Izard, 1972)
The scale comprises 30 adjectives (or items) and participants were required to rate on a 5-point Likert scale the extent to which each word described the way s/he felt at the current time (1 = “not at all” and 5 = “very strongly”). The sub-scales sadness and anger were collapsed into a Negative Emotion scale.
Statistical Analyses
All statistical analyses were performed using SPSS software (SPSS Inc., Version 21, Chicago, IL, USA). Figures were created with GraphPad Prism 7 (GraphPad Software Inc., San Diego, CA, USA). Between Factors of Group (Moderate social drinkers vs Problem drinkers), and Within Factors of Imagery Condition (Stress, Neutral, Alcohol cue) and Time-points (baseline, response to imagery, response to discrete alcohol cue) represented the Fixed Effect factors. Participants were the Random Effect factor. A Compound Symmetry covariance structure was applied to all models. Standard regression analyses were also performed in order to assess whether cytokine adaptations were predictive of alcohol craving and alcohol motivation and intake. A series of t-tests and chi-square analyses were conducted to examine any group variation in demographics. Bonferroni tests were used as adjustments for multiple comparisons.
RESULTS
Participants
Of 64 subjects, 26 met criteria for problem drinking (> 8 on the AUDIT for men; >7 on the AUDIT for women). Thirty eight met criteria for moderate social drinking (≤ 8 on the AUDIT for men; ≤ 7 on the AUDIT for women). Participants were predominantly non-smoking with only two smokers in the problem drinking group and one smoker in the social drinking group. Both groups of non-dependent drinkers were statistically matched for all demographic factors with the exception of quantity and frequency of alcohol use (see Table 1).
Table 1.
Demographics Table
| Social Drinkers N=38 |
Problem Drinkers N=26 |
|
|---|---|---|
|
| ||
| AUDIT score | 4.1 ± 5.0 | 13.6 ± 4.2 |
|
| ||
| Age | 30.1 ± 7.9 | 27.2 ± 6.8 |
|
| ||
| Years of Education | 15.5 ± 1.7 | 16.0 ± 2.3 |
|
| ||
| Gender - % males | 68.4% (n=26) | 80.8% (n=21) |
|
| ||
| Race – Caucasian | 63.1% | 80.8% |
| African American | 21.1% | 11.5% |
| Hispanic | 5.3% | 0 |
| Asian | 7.9% | 3.8% |
| Other | 2.6% | 3.8% |
|
| ||
| No. of years drinking | 5.3 ± 5.0 | 8.5 ± 6.5 |
|
| ||
| No. of drinks in last month | 24.0 ± 25.7 | 82.5 ± 71.8 |
|
| ||
| No. of smokers | 1 | 2 |
|
| ||
| No. meeting criteria for lifetime mood disorder | 3 | 1 |
|
| ||
| No. meeting criteria for current mood disorder | 1 | 0 |
|
| ||
| No. meeting criteria for lifetime anxiety disorder | 1 | 0 |
|
| ||
| No. meeting criteria for current anxiety disorder | 0 | 0 |
Note. Shaded area: p≤.05
Immune System Measures
Interleukin (IL-6)
Differences between Problem and Moderate Social Drinkers in Interleukin-6 (IL-6)
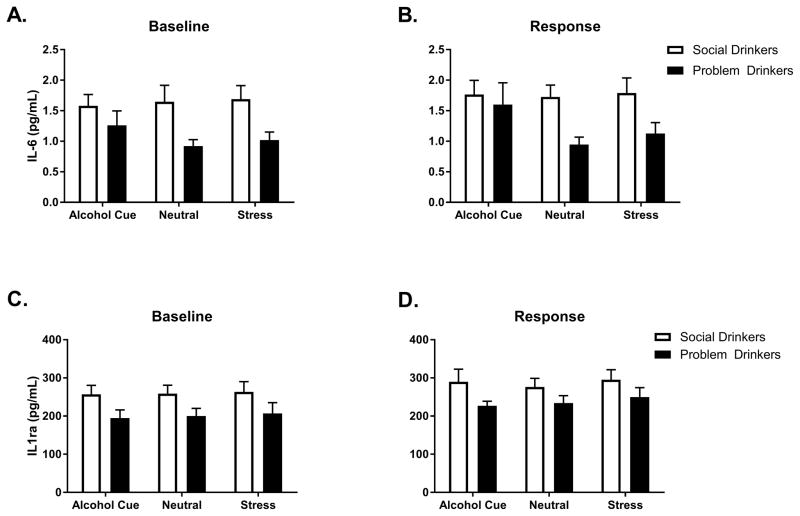
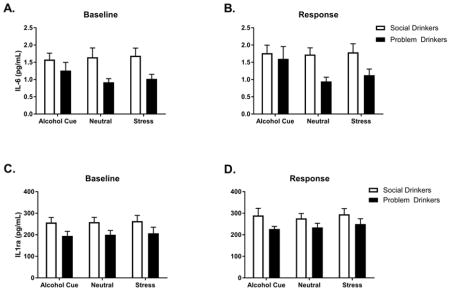
A main effect of Group at baseline, [F(1, 61) = 8.5, p=.005] indicated that tonic levels of IL-6 were significantly lower in the problem drinkers compared with the moderate social drinkers, across all 3 consecutive days (Fig. 1a). Following exposure to all 3 imagery conditions, the main effect of Group was still observed [F(1, 61) =6.3, p<.02], again showing attenuated levels of IL-6 in the problem drinking group compared with the moderate social drinkers (Fig. 1b). In order to ascertain the extent to which these IL-6 changes were a function of tonic adaptations, a second analysis was conducted with the baseline included as a covariate. After controlling for baseline variation, no significant differences in response to imagery conditions between groups were observed.
Fig. 1.
IL-6 levels at (A) BASELINE and (B) in RESPONSE to stress, neutral and alcohol cue guided imagery in problem and social drinkers. IL-6 levels were lower in problem compared to social drinkers across all conditions. IL-1ra levels at (C) BASELINE and (D) in RESPONSE to stress, neutral and alcohol cue guided imagery in problem and social drinkers. IL-1ra levels were lower in problem compared to social drinkers across all conditions. Data are displayed as mean ± S.E.M.
Interleukin-1 receptor antagonist (IL-1ra)
Differences between Problem and Moderate Social Drinkers in Interleukin-1 receptor antagonist (IL-1ra)
A main effect of Group at baseline, [F(1, 61) = 4.8, p=.03] indicated that tonic levels of IL-1ra were also attenuated in the problem drinkers compared with the moderate social drinkers, across all 3 consecutive days (Fig. 1c). Following exposure to all 3 imagery conditions, a significant main effect of Group [F(1, 62) = 4.5, p<.04] again indicated attenuation in the problem drinkers compared with the controls across all three imagery conditions (Fig. 1d). In order to examine whether this phasic down-regulation was a function of tonic adaptations, a second analysis was conducted including the baseline as a covariate. A significant Group X Imagery condition interaction [F(2, 268) = 4.4, p=.01] indicated that problem drinkers continued to demonstrate an attenuated IL-1ra response following exposure to the cue condition (p=.03).
Tumor Necrosis Factor-alpha (TNFα)
Differences between Problem and Moderate Social Drinkers in Tumor Necrosis Factor-alpha (TNFα)
No significant main effects and/or interactions were observed for TNFα levels, either at baseline or following imagery exposure.
Subjective Measures
Alcohol Craving and Intake
Alcohol Craving
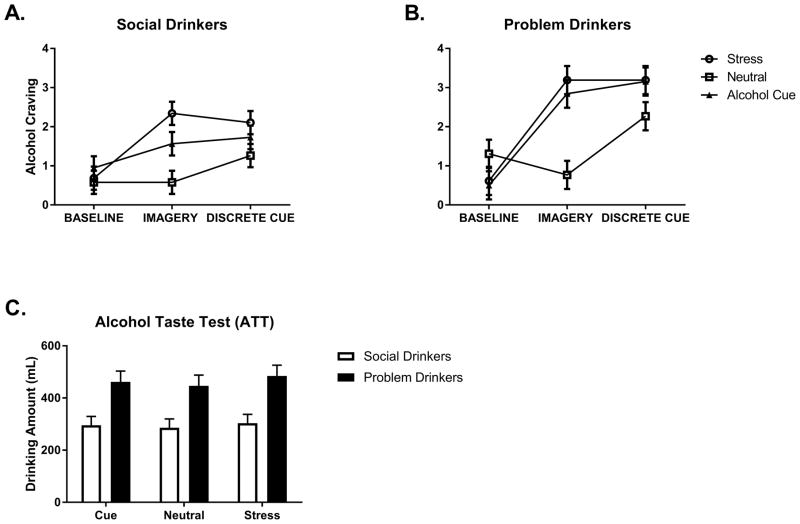
Following exposure to all 3 imagery conditions, a significant Group X Condition X Time-point interaction was observed for alcohol craving, [F(4, 494) = 3.2, p=.01] (Fig. 2a and b). This indicated that the problem drinkers reported significantly elevated alcohol craving across all three imagery conditions at the third time-point, following the presentation of beer, compared with the moderate social drinkers (Stress: p=.003; Cue: p=.02; Neutral: p=.03). In addition, problem drinkers reported higher craving at the second time-point immediately following stress imagery exposure compared with the moderate social drinkers (p=.007). No group differences were observed at baseline. While both groups reported significantly higher craving in the cue compared with the neutral condition immediately following imagery exposure (Problem Drinkers: Cue>Neutral, p<.0001; Moderate Social Drinkers: Cue>Neutral, p<.0001) and following presentation of beer (Problem Drinkers: Cue>Neutral, p= .03; Moderate Social Drinkers: Cue>Neutral, p=.01) only the problem drinkers reported higher craving following stress imagery, both immediately after imagery presentation (Stress>Neutral, p<.0001) and 10 minutes later following presentation of the beer (Stress>Neutral, p=.04).
Fig. 2.
Alcohol craving in (A) social drinkers and (B) problem drinkers as a function of timepoint. (C) Problem drinkers consumed higher amounts of beer following the presentation of beer cues compared to social drinkers in response to all three imagery conditions. Data are displayed as mean ± S.E.M.
Alcohol Taste Test
Following exposure to all three imagery conditions and a discrete alcohol cue, the problem drinkers consumed significantly more beer during the alcohol taste test compared with the moderate social drinkers [F(1, 62) = 11.5, p=.001] (Fig. 2c).
Mood and Anxiety
Anxiety
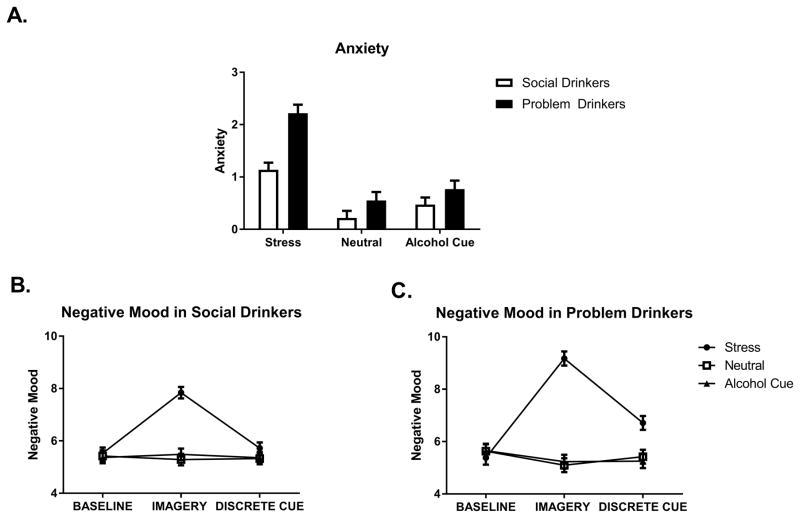
A significant main effect of Group [F(1, 62) = 11.6, p<.0001] showed that the problem drinkers reported elevated anxiety compared with the moderate social drinkers across all imagery conditions and time-points. An additional Group X Condition interaction, [F(2, 493) = 7.5, p=.001] further indicated that this interaction effect was due to the response to the stress imagery condition, where the problem drinkers reported significantly higher anxiety compared with the moderate social drinkers (p<.0001) (See Fig. 3a). As anticipated, both groups reported higher anxiety following stress compared with both the neutral imagery conditions (p<.0001, in all cases).
Fig. 3.
(A) Anxiety in social drinkers and problem drinkers. Problem drinkers reported higher anxiety than social drinkers across all imagery conditions and timepoints. Negative mood in (B) social drinkers and (C) problem drinkers as a function of timepoint. Problem drinkers reported higher ratings of negative mood following exposure to stress imagery, immediately following stress exposure and following beer cue presentation. Data are displayed as mean ± S.E.M.
Negative Mood (Sadness, Anger-DES)
A significant Group X Imagery Condition X Time-point interaction [F(4, 494) = 3.7, p=.006] indicated that the problem drinkers reported higher ratings of negative mood following exposure to stress imagery compared with the moderate social drinkers, both immediately following stress exposure (p<.0001) and 10 minutes later following beer presentation (p<.0001). While both groups reported higher negative mood in the stress compared with the cue and neutral conditions immediately following imagery presentation (p<.0001, in all cases), only the problem drinkers continued to report elevated negative mood 10 minutes later following exposure to the discrete alcohol cue, compared with both the cue (p<.0001) and neutral (p<.0001) conditions (Fig. 3b and c).
Cytokine Alterations and Prediction of Alcohol Motivation and Intake
IL-6 and Alcohol Motivation and Intake
Standard linear regression models indicated that basal levels of IL-6 were significant predictors of IL-6 response immediately following imagery exposure in all three imagery conditions (Stress: β= 0.9, t = 14.6, p < 0.0001), (Cue: β= 1.03, t = 18.8, p < 0.0001), (Neutral: β= 0.8, t = 10.1, p < 0.0001). Positive beta values showed that attenuated levels of IL-6 at baseline predicted attenuated levels of IL-6 following imagery exposure.
Basal levels of IL-6 also predicted elevations in alcohol craving immediately following stress (β= −2.5, t = −2.4, p < 0.02), and cue (β= −2.0, t = −2.0, p < 0.05) exposure, but not after exposure to the neutral imagery condition. Negative beta values indicated that greater attenuation of IL-6 at baseline predicted higher alcohol craving following stress and cue exposure. Low basal levels of IL-6 also predicted increased alcohol craving following stress exposure at the 10 minute recovery time-point following the presentation of beer (β= −2.8, t = −3.0, p = 0.004).
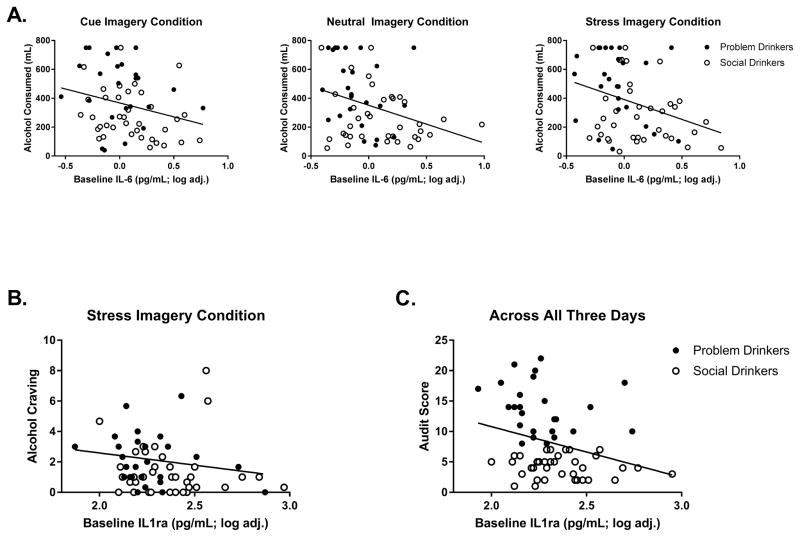
Basal levels of IL-6 also predicted elevations in total amount of alcohol consumed during the taste test following exposure to all three imagery conditions: (Stress: β= −275.5, t = −2.6, p = 0.01), (Cue: β= −192.4, t = −2.1, p = 0.04), (Neutral: β= −261.1, t = −2.7 p = 0.008) (Fig. 4a). Again negative beta values indicated that the lower the levels of IL-6 at baseline, the greater amount of alcohol consumed during the taste test and following imagery exposure. Similarly, attenuated basal levels of IL-6 also predicted drinking behaviors following exposure to stress, including a greater number of times the participants either touched (β= −13.2, t = −3.1, p = 0.003) or picked up (β= −9.4, t = −2.6, p = 0.01) the beer glasses as well as a higher number of sips taken (β= −9.1, t = −2.7, p = 0.008). Following exposure to the cue condition, low baseline levels of IL-6 predicted a greater number of times the participants touched the glasses (β= −8.5, t = −2.3, p < 0.03) or picked up the glasses (β= −6.8, t = −2.1, p = 0.04).
Fig. 4.
Regression analyses across both groups, with illustration of group distributions for Problem Drinkers (closed circle) and Social Drinkers (open circle). (A) Basal levels of IL-6 predict elevations in total amount of alcohol consumed during the alcohol taste test following exposure to all three imagery conditions. (B) Basal levels of IL-1ra predict elevations in alcohol craving immediately following exposure to stress imagery. (C) Basal levels of IL-1ra predict AUDIT scores across all 3 days.
IL-6 and Problem Drinking Measure
Basal levels of IL-6 across 2 of the 3 consecutive experimental days predicted AUDIT scores (Day 1: β= −6.9, t = −2.8, p = 0.007), (Day 2: β= −7.0, t = −2.9, p = 0.005), (Day 3: β= −2.6, t = −1.0, p = ns). Again, negative beta values showed that the lower the basal IL-6 levels, the greater the severity of problem drinking (as characterized by higher AUDIT scores).
IL-1ra Changes and Alcohol Motivation and Intake
Standard linear regression models indicated that basal levels of IL-1ra were significant predictors of IL-1ra response immediately following imagery exposure in all three imagery conditions (Stress: β= 0.82, t = 12.6, p < 0.0001), (Cue: β= 0.89, t = 14.4, p < 0.0001), (Neutral: β= 0.90, t = 14.6, p < 0.0001). Positive beta values showed that attenuated levels of IL1-ra at baseline predicted attenuated levels of IL-1ra following imagery exposure.
Basal levels of IL-1ra predicted elevations in alcohol craving immediately following exposure to stress imagery (β= −2.5, t = −1.9, p = 0.05) (Fig. 4b) but not following exposure to cue or neutral imagery. Again, negative beta values indicated that greater attenuation of basal IL-1ra predicted a higher reported alcohol craving following stress. Basal levels of IL-1ra did not predict either level of alcohol consumption or drinking behaviors following exposure to imagery.
IL-1ra and Problem Drinking Measure
Basal levels of IL-1ra predicted AUDIT scores across all 3 days (Day 1: β= −8.2, t = −2.4, p < 0.02), (Day 2: β= −7.2, t = −2.2, p = 0.03), (Day 3: β= −8.8, t = −2.4, p < 0.02). As such, basal levels of IL-1ra averaged across all 3 days predicted AUDIT scores (β= −8.3, t = −2.9, p < 0.02) (Fig. 4c). The lower the basal IL-1ra levels, the greater the AUDIT scores.
Tumor Necrosis Factor-alpha (TNFα) and Alcohol Motivation and Intake
Tumor Necrosis Factor-alpha (TNFα)
baseline or response measure was not predictive of subjective alcohol craving or behavioral alcohol motivation and intake.
DISCUSSION
Findings from the current study show that problem drinkers potentially “at risk” for alcohol dependence demonstrated suppressed pro- and anti-inflammatory markers at baseline compared with moderate social drinkers. This suppression persisted following exposure to stress, cue and a discrete alcohol cue despite problem drinkers reporting elevated levels of stress-induced anxiety, negative mood and craving. Notably, this dampened biological response was predominantly a function of diminished immune system tone. In support of this, basal changes in pro-inflammatory IL-6 predicted not only an elevated desire for alcohol following stress and cue imagery, but also subsequent behavioral alcohol motivation assessed by the amount of alcohol consumed in the ATT. Similarly, basal changes in the anti-inflammatory marker IL-1ra also predicted elevated craving for alcohol following stress. This suggests that peripheral cytokines may play a motivational role in the development of addictive behaviors characterized in the current study by subjective stress-induced and cue-induced craving as well as objective levels of alcohol consumption. While future research is encouraged in order to more fully understand the allostatic mechanisms driving this down-regulation in both pro- and anti-inflammatory markers, preliminary data from the current study does suggest that tonic and phasic suppression in selective peripheral cytokine markers may represent salient indicators of risk in vulnerable drinkers.
As low levels of both IL-1ra and IL-6 were associated with craving and drinking in the current study, such changes may reflect a chronic inflammatory state. As IL-1ra competitively binds to the IL-1 cell surface receptor and counteracts the inflammatory effects of IL-1, low levels may be an indirect sign of increased peripheral inflammatory activity in the current problem drinkers (Charles et al., 2011). Moreover, this shows some consistency with prior studies that have documented an increased peripheral pro-inflammatory state via changes in similar cytokine markers, within alcohol dependent individuals. For example, attenuated levels of the anti-inflammatory Interleukin-10 (IL-10) have been observed following early abstinence in cocaine dependent individuals (Fox et al., 2012), alcoholics free of liver pathology (Gonzalez-Quintela et al., 2000) and abstinent alcoholics without anxiety and/or mood disorders (Fox et al., 2010b). Of note, IL-10 levels were not obtained in the current study of non-dependent social and problem drinkers and should be examined in future studies.
With regard to attenuated levels of IL-6, the interpretation of a chronic inflammatory state is far more complex due to the fact that IL-6 mediates both pro- and anti-inflammatory processes. While the role of IL-6 as the primary stimulator of acute phase proteins is well documented (Volpato et al., 2004), inducible IL-6 has also been shown to down-regulate levels of pro-inflammatory markers and stimulate anti-inflammatory cytokines (Gabay, 2006). Prolonged activation of IL-6 has also been shown to mimic the anti-inflammatory effects of IL-10 (Yasukawa et al., 2003). The pre-clinical literature also provides some support for the suppression of IL-6 by alcohol showing acute ethanol exposure to suppress IL-6 production by alveolar macrophages in response to endotoxin (Karavitis et al., 2008), and further, that binge drinking models in mice suppress serum levels of IL-6 (Pruett et al., 2004, Bhatty et al., 2011). Conversely, while many clinical studies have typically shown affective disorders to be characterized by high levels of IL-6, it is worth noting that the problem drinking group did not meet criteria for current mood and anxiety disorders, suggesting that the relative contribution of drinking and mood on IL-6 adaptations may be very different.
It is also important to note that no tonic or phasic adaptations in the pro-inflammatory cytokine TNFα were observed in problem, compared with moderate social drinkers. Much prior research has shown that elevations in TNFα are particularly associated with the development and regulation of deleterious mood in both laboratory animals and clinical populations. Pathological activation of TNFα associated with depressive symptoms in chronically ill patients (Maes et al., 2009, McNally et al., 2008). Other methodological paradigms have also produced findings showing that the treatment of both patients (Dunn et al., 2005) and animals (Silverman et al., 2007, Salome et al., 2008) with pro-inflammatory cytokines, such as TNFα produces depressive and deleterious moods similar to those known to underlie the stress-induced alcohol craving state (Litt and Cooney, 1999, Fox et al., 2007). Again, one tentative explanation for the current findings in problem drinkers may be that elevated TNFα, similar to elevated IL-6, is associated selectively with the mood-related aspects of craving, which are sensitized to a greater extent during early abstinence in dependent, rather than non-dependent problem individuals.
Current findings also show that problem drinkers demonstrate a stress-related craving state similar, but less robust, to that observed in early abstinent alcoholics, and which is defined by sensitized negative mood and anxiety compared with socially drinking controls (Fox et al., 2007, Sinha et al., 2009, Sinha et al., 2011). Furthermore, in contrast to previous findings in abstinent alcoholics, the current problem drinkers demonstrated an alcohol cue-related craving state that was not characterized by elevations in either anxiety or negative mood. For example, the problem drinkers reported no negative emotional or anxiety response to the alcohol cue condition, which commonly reflects a variant stressor in dependent individuals. In this case, findings correspond more to prior results seen in moderate and social drinkers (Sinha et al., 2009, Sinha et al., 2011), where the cue imagery condition does not induce negative emotions. While variation in the anxiety response to stress may reflect severity of alcohol consumption, the qualitatively different response to alcohol cue imagery is more likely related to the continued use of alcohol in the current problem drinkers. In previous studies examining the nature of alcohol cue response in early abstinent alcohol dependent individuals, the desire for abstinence alongside alcohol cue imagery and beer cues are likely to create conflicting negative appetitive goals that are not as pertinent to active drinkers. Interestingly then, attenuation of immune system markers may highlight a mechanism underpinning both stress-related and reward-related motivation for alcohol in actively drinking vulnerable populations.
Although the current study only examined peripheral levels of pro-inflammatory (TNFα, IL-6) and anti-inflammatory (IL-1ra) cytokines, these markers are known to readily cross the blood brain barrier (Pan et al., 2011, Banks, 2005), and hence can serve as indirect markers of brain levels. Moreover, these immune system cytokines are strongly linked with mood related disorders (Eyre et al., 2016, Hayley, 2011, Abbott et al., 2015) and chronic peripheral immune system adaptations may impinge upon central neural systems that underlie regulatory function, goal-oriented behaviors and impulse control. Again, while the precise mechanisms are not well understood, acute artificially-induced peripheral inflammation has been shown to increase deleterious mood and also to exert adaptations within regions of the prefrontal cortex implicated in inhibitory regulation (Harrison et al., 2009; Hannestad et al., 2012b). Similarly, inflammation-induced poor mood change has been associated with reduced connectivity of the sub-genular anterior cingulate to amygdala, medial prefrontal cortex, and nucleus accumbens (Harrison et al., 2009), brain regions important in addiction. While the current study has observed a dampening of peripheral cytokines, these include anti-inflammatory markers. Hence, a global and complex adaptation of the immune system in problem drinkers could be altering central nervous system functioning that regulates mood and inhibitory control, and therefore directly affecting alcohol-related behaviors.
While this is one of the first studies to characterize immune system changes underlying stress- and cue-related alcohol craving in problem drinkers, some caution must be taken with regard to interpretation, due to methodological limitations. First, while the current study has shown that immune system adaptations may predict provoked craving and drinking within the laboratory, more research is clearly needed to assess whether these changes additionally extend to predicting clinical outcome in problem drinkers, beyond the laboratory. This may include assessing whether peripheral cytokines predict lapse to alcohol, drinking severity and/or abstinence in individuals wanting to quit. Second, systematically examining immune system adaptations in problem drinkers following incremental periods of abstinence may also be required to provide a more complete assessment of cytokine adaptations with regard to their function in addiction risk and recovery. Also, a better understanding is needed of whether this altered immune system state in problem drinkers reflects an underlying predisposition to drinking or whether it is an outcome of high drinking levels. Third, while both groups were statistically matched for number of men and women in the present study, important sex differences may exist in peripheral cytokines in problem drinkers. As sex-specific differences are observed during early abstinence from alcohol and drugs, as well as in the development of addiction, there is a need to more thoroughly examine sex differences in a larger sample of male and female social and problem drinkers. Lastly, although it is important to look at cytokines as markers of drinking, it would be important to understand more specifically the mechanisms that underlie these processes. Despite these limitations, this study highlights important immune system aspects of stress- and cue-related motivation for alcohol and drinking in non-dependent problems drinkers. While, similar to alcoholics, problem drinkers show a sensitized craving response to both stress and alcohol cue, unlike abstinent alcoholics they demonstrate less sensitized anxiety to stress, and no negative mood response or anxiety to alcohol cue. They do, however show robust adaptations to tonic levels of specific peripheral cytokines including IL-6 and IL-1ra, which predict both stress and reward related aspects of craving and drinking. As such, selective peripheral cytokines may reflect efficacious targets for therapeutic intervention in “at risk” problem drinkers.
Acknowledgments
We would like to thank the staff at the Yale Stress Center and the Yale Center for Clinical Investigation. We would also like to thank Dr. Keri Tuit Height and Dr. Joseph Guarnaccia for their assistance in completing this study. This study was supported in part by grants R01: AA 20095 (Fox), R03:AA022500 (Fox), Peter F MacManus Charitable Trust (Fox), R01: AA 20504 (Sinha), R01: AA013892 (Sinha), U1DE019586 (Sinha), UL1 TR000142 (Yale CTSA) and 5T32DA007238-24 (Milivojevic).
Footnotes
CONFLICT OF INTEREST
All authors declare that they have no competing financial interests pertaining to the aims and results of this study.
References
- ABBOTT R, WHEAR R, NIKOLAOU V, BETHEL A, COON JT, STEIN K, DICKENS C. Tumour necrosis factor-alpha inhibitor therapy in chronic physical illness: A systematic review and meta-analysis of the effect on depression and anxiety. J Psychosom Res. 2015;79:175–84. doi: 10.1016/j.jpsychores.2015.04.008. [DOI] [PubMed] [Google Scholar]
- BABOR TF, HIGGINS-BIDDLE JC, SAUNDERS JB, MONTEIRO MG. The Alcohol Use Disorders Identification Test: Guidelines for Use in Primary Care. World Health Organization Department of Mental Health and Substance Dependence; 2001. [Google Scholar]
- BANKS WA. Blood-brain barrier transport of cytokines: a mechanism for neuropathology. Curr Pharm Des. 2005;11:973–84. doi: 10.2174/1381612053381684. [DOI] [PubMed] [Google Scholar]
- BAUNE BT, DANNLOWSKI U, DOMSCHKE K, JANSSEN DG, JORDAN MA, OHRMANN P, BAUER J, BIROS E, AROLT V, KUGEL H, BAXTER AG, SUSLOW T. The interleukin 1 beta (IL1B) gene is associated with failure to achieve remission and impaired emotion processing in major depression. Biol Psychiatry. 2010;67:543–9. doi: 10.1016/j.biopsych.2009.11.004. [DOI] [PubMed] [Google Scholar]
- BEECH RD, LEFFERT JJ, LIN A, HONG KA, HANSEN J, UMLAUF S, MANE S, ZHAO H, SINHA R. Stress-related alcohol consumption in heavy drinkers correlates with expression of miR-10a, miR-21, and components of the TAR-RNA-binding protein-associated complex. Alcohol Clin Exp Res. 2014;38:2743–53. doi: 10.1111/acer.12549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BHATTY M, JAN BL, TAN W, PRUETT SB, NANDURI B. Role of acute ethanol exposure and TLR4 in early events of sepsis in a mouse model. Alcohol. 2011;45:795–803. doi: 10.1016/j.alcohol.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BIENKOWSKI MS, RINAMAN L. Immune challenge activates neural inputs to the ventrolateral bed nucleus of the stria terminalis. Physiol Behav. 2011;104:257–65. doi: 10.1016/j.physbeh.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLAINE S, SEO D, SINHA R. Craving, cortisol, and compulsion: neuroendocrine and neural dysfunction influences on binge drinking. Alcoholism: Clinical and Experimental Research. 2016;40(S1):216A. [Google Scholar]
- BLUME AW, MARLATT GA. The role of executive cognitive functions in changing substance use: what we know and what we need to know. Ann Behav Med. 2009;37:117–25. doi: 10.1007/s12160-009-9093-8. [DOI] [PubMed] [Google Scholar]
- BRADLEY KA, WICKIZER JB, POWELL SH, BURMAN ML. Alcohol Screening Questionnaires in Women: A critical review. JAMA. 2003;280(2):166–71. doi: 10.1001/jama.280.2.166. [DOI] [PubMed] [Google Scholar]
- CAUDILL BD, MARLATT GA. Modeling influences in social drinking: an experimental analogue. J Consult Clin Psychol. 1975;43:405–15. doi: 10.1037/h0076689. [DOI] [PubMed] [Google Scholar]
- CHARLES BA, DOUMATEY A, HUANG H, ZHOU J, CHEN G, SHRINER D, ADEYEMO A, ROTIMI CN. The roles of IL-6, IL-10, and IL-1RA in obesity and insulin resistance in African-Americans. J Clin Endocrinol Metab. 2011;96:E2018–22. doi: 10.1210/jc.2011-1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHRISTENSEN MV, KESSING LV. The hypothalamo-pituitary-adrenal axis in major affective disorder: a review. Nord J Psychiatry. 2001;55:359–63. doi: 10.1080/080394801317080873. [DOI] [PubMed] [Google Scholar]
- CRAIG AD. Interoception: the sense of the physiological condition of the body. Curr Opin Neurobiol. 2003;13:500–5. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- DAEPPEN JB, YERSIN B, LANDRY U, PECOUD A, DECREY H. Reliability and validity of the Alcohol Use Disorders Identification Test (AUDIT) imbedded within a general health risk screening questionnaire: results of a survey in 332 primary care patients. Alcohol Clin Exp Res. 2000;24:659–65. [PubMed] [Google Scholar]
- DUNN AJ, SWIERGIEL AH, DE BEAUREPAIRE R. Cytokines as mediators of depression: what can we learn from animal studies? Neurosci Biobehav Rev. 2005;29:891–909. doi: 10.1016/j.neubiorev.2005.03.023. [DOI] [PubMed] [Google Scholar]
- ENGLER H, DOENLEN R, ENGLER A, RIETHER C, PRAGER G, NIEMI MB, PACHECO-LOPEZ G, KRUGEL U, SCHEDLOWSKI M. Acute amygdaloid response to systemic inflammation. Brain Behav Immun. 2011;25:1384–92. doi: 10.1016/j.bbi.2011.04.005. [DOI] [PubMed] [Google Scholar]
- EYRE HA, LAVRETSKY H, KARTIKA J, QASSIM A, BAUNE BT. Modulatory Effects of Antidepressant Classes on the Innate and Adaptive Immune System in Depression. Pharmacopsychiatry. 2016 doi: 10.1055/s-0042-103159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FIRST M, SPITZER R, GIBBON M, WILLIAMS J. Structured Clinical Interview for DSM-IV. Patient Edition. Washington DC: American Psychiatric Press Inc; 1995. [Google Scholar]
- FOX HC, BERGQUIST KL, HONG KI, SINHA R. Stress-induced and alcohol cue-induced craving in recently abstinent alcohol-dependent individuals. Alcohol Clin Exp Res. 2007;31:395–403. doi: 10.1111/j.1530-0277.2006.00320.x. [DOI] [PubMed] [Google Scholar]
- FOX HC, BERGQUIST KL, PEIHUA G, RAJITA S. Interactive effects of cumulative stress and impulsivity on alcohol consumption. Alcohol Clin Exp Res. 2010a;34:1376–85. doi: 10.1111/j.1530-0277.2010.01221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOX HC, D’SA C, SINHA R. Altered cytokine responses are associated with enhanced stress-induced craving in alcohol dependent individuals. Alcohol Clin Exp Res. 2010b;34(6):143. [Google Scholar]
- FOX HC, D’SA C, KIMMERLING A, SIEDLARZ KM, TUIT KL, STOWE R, SINHA R. Immune system inflammation in cocaine dependent individuals: implications for medications development. Hum Psychopharmacol. 2012;27:156–66. doi: 10.1002/hup.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOX HC, TALIH M, MALISON R, ANDERSON GM, KREEK MJ, SINHA R. Frequency of recent cocaine and alcohol use affects drug craving and associated responses to stress and drug-related cues. Psychoneuroendocrinology. 2005;30:880–91. doi: 10.1016/j.psyneuen.2005.05.002. [DOI] [PubMed] [Google Scholar]
- GABAY C. Interleukin-6 and chronic inflammation. Arthritis Res Ther. 2006;8(Suppl 2):S3. doi: 10.1186/ar1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GONZALEZ-QUINTELA A, MELLA C, PEREZ LF, ABDULKADER I, CAPARRINI AM, LOJO S. Increased serum tissue polypeptide specific antigen (TPS) in alcoholics: a possible marker of alcoholic hepatitis. Alcohol Clin Exp Res. 2000;24:1222–6. [PubMed] [Google Scholar]
- HALME JT, SEPPA K, ALHO H, PIRKOLA S, POIKOLAINEN K, LONNQVIST J, AALTO M. Hazardous drinking: prevalence and associations in the Finnish general population. Alcohol Clin Exp Res. 2008;32:1615–22. doi: 10.1111/j.1530-0277.2008.00740.x. [DOI] [PubMed] [Google Scholar]
- HANNESTAD J, GALLEZOT JD, SCHAFBAUER T, LIM K, KLOCZYNSKI T, MORRIS ED, CARSON RE, DING YS, COSGROVE KP. Endotoxin-induced systemic inflammation activates microglia: [(1)(1)C]PBR28 positron emission tomography in nonhuman primates. Neuroimage. 2012a;63:232–9. doi: 10.1016/j.neuroimage.2012.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANNESTAD J, SUBRAMANYAM K, DELLAGIOIA N, PLANETA-WILSON B, WEINZIMMER D, PITTMAN B, CARSON RE. Glucose metabolism in the insula and cingulate is affected by systemic inflammation in humans. J Nucl Med. 2012b;53:601–7. doi: 10.2967/jnumed.111.097014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARRISON NA, BRYDON L, WALKER C, GRAY MA, STEPTOE A, CRITCHLEY HD. Inflammation causes mood changes through alterations in subgenual cingulate activity and mesolimbic connectivity. Biol Psychiatry. 2009;66:407–14. doi: 10.1016/j.biopsych.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAYLEY S. Toward an anti-inflammatory strategy for depression. Front Behav Neurosci. 2011;5:19. doi: 10.3389/fnbeh.2011.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- INAGAKI TK, MUSCATELL KA, IRWIN MR, COLE SW, EISENBERGER NI. Inflammation selectively enhances amygdala activity to socially threatening images. Neuroimage. 2012;59:3222–6. doi: 10.1016/j.neuroimage.2011.10.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IVIS FJ, ADLAF EM, REHM J. Incorporating the AUDIT into a general population telephone survey: a methodological experiment. Drug Alcohol Depend. 2000;60:97–104. doi: 10.1016/s0376-8716(99)00145-3. [DOI] [PubMed] [Google Scholar]
- IZARD C. Patterns of emotions: A new analysis of anxiety and depression. New York: Academic Press; 1972. [Google Scholar]
- KARAVITIS J, MURDOCH EL, GOMEZ CR, RAMIREZ L, KOVACS EJ. Acute ethanol exposure attenuates pattern recognition receptor activated macrophage functions. J Interferon Cytokine Res. 2008;28:413–22. doi: 10.1089/jir.2007.0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANG PJ, KOZAK MJ, MILLER GA, LEVIN DN, MCLEAN A., JR Emotional imagery: conceptual structure and pattern of somato-visceral response. Psychophysiology. 1980;17:179–92. doi: 10.1111/j.1469-8986.1980.tb00133.x. [DOI] [PubMed] [Google Scholar]
- LANG PJ, LEVIN DN, MILLER GA, KOZAK MJ. Fear behavior, fear imagery, and the psychophysiology of emotion: the problem of affective response integration. J Abnorm Psychol. 1983;92:276–306. doi: 10.1037//0021-843x.92.3.276. [DOI] [PubMed] [Google Scholar]
- LASO FJ, IGLESIAS-OSMA C, CIUDAD J, LOPEZ A, PASTOR I, ORFAO A. Chronic alcoholism is associated with an imbalanced production of Th-1/Th-2 cytokines by peripheral blood T cells. Alcohol Clin Exp Res. 1999;23:1306–11. [PubMed] [Google Scholar]
- LASO FJ, VAQUERO JM, ALMEIDA J, MARCOS M, ORFAO A. Production of inflammatory cytokines by peripheral blood monocytes in chronic alcoholism: relationship with ethanol intake and liver disease. Cytometry B Clin Cytom. 2007;72:408–15. doi: 10.1002/cyto.b.20169. [DOI] [PubMed] [Google Scholar]
- LITT MD, COONEY NL. Inducing craving for alcohol in the laboratory. Alcohol Res Health. 1999;23:174–8. [PMC free article] [PubMed] [Google Scholar]
- MAES M, YIRMYIA R, NORABERG J, BRENE S, HIBBELN J, PERINI G, KUBERA M, BOB P, LERER B, MAJ M. The inflammatory & neurodegenerative (I&ND) hypothesis of depression: leads for future research and new drug developments in depression. Metab Brain Dis. 2009;24:27–53. doi: 10.1007/s11011-008-9118-1. [DOI] [PubMed] [Google Scholar]
- MARCOS M, PASTOR I, GONZALEZ-SARMIENTO R, LASO FJ. Interleukin-10 gene polymorphism is associated with alcoholism but not with alcoholic liver disease. Alcohol Alcohol. 2008;43:523–8. doi: 10.1093/alcalc/agn026. [DOI] [PubMed] [Google Scholar]
- MCNALLY L, BHAGWAGAR Z, HANNESTAD J. Inflammation, glutamate, and glia in depression: a literature review. CNS Spectr. 2008;13:501–10. doi: 10.1017/s1092852900016734. [DOI] [PubMed] [Google Scholar]
- NIAAA. Helping patients who drink too much a clinicians guide 2005 [Google Scholar]
- PAN W, STONE KP, HSUCHOU H, MANDA VK, ZHANG Y, KASTIN AJ. Cytokine signaling modulates blood-brain barrier function. Curr Pharm Des. 2011;17:3729–40. doi: 10.2174/138161211798220918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRUETT SB, ZHENG Q, FAN R, MATTHEWS K, SCHWAB C. Ethanol suppresses cytokine responses induced through Toll-like receptors as well as innate resistance to Escherichia coli in a mouse model for binge drinking. Alcohol. 2004;33:147–55. doi: 10.1016/j.alcohol.2004.08.001. [DOI] [PubMed] [Google Scholar]
- SALOME N, TASIEMSKI A, DUTRIEZ I, WIGGER A, LANDGRAF R, VILTART O. Immune challenge induces differential corticosterone and interleukin-6 responsiveness in rats bred for extremes in anxiety-related behavior. Neuroscience. 2008;151:1112–8. doi: 10.1016/j.neuroscience.2007.12.010. [DOI] [PubMed] [Google Scholar]
- SERRATS J, SAWCHENKO PE. CNS activational responses to staphylococcal enterotoxin B: T-lymphocyte-dependent immune challenge effects on stress-related circuitry. J Comp Neurol. 2006;495:236–54. doi: 10.1002/cne.20872. [DOI] [PubMed] [Google Scholar]
- SILVERMAN MN, MACDOUGALL MG, HU F, PACE TW, RAISON CL, MILLER AH. Endogenous glucocorticoids protect against TNF-alpha-induced increases in anxiety-like behavior in virally infected mice. Mol Psychiatry. 2007;12:408–17. doi: 10.1038/sj.mp.4001921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SINHA R. How does stress increase risk of drug abuse and relapse? Psychopharmacology (Berl) 2001;158:343–59. doi: 10.1007/s002130100917. [DOI] [PubMed] [Google Scholar]
- SINHA R. The role of stress in addiction relapse. Curr Psychiatry Rep. 2007;9:388–95. doi: 10.1007/s11920-007-0050-6. [DOI] [PubMed] [Google Scholar]
- SINHA R, FOX HC, HONG KA, BERGQUIST K, BHAGWAGAR Z, SIEDLARZ KM. Enhanced negative emotion and alcohol craving, and altered physiological responses following stress and cue exposure in alcohol dependent individuals. Neuropsychopharmacology. 2009;34:1198–208. doi: 10.1038/npp.2008.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SINHA R, FOX HC, HONG KI, HANSEN J, TUIT K, KREEK MJ. Effects of adrenal sensitivity, stress- and cue-induced craving, and anxiety on subsequent alcohol relapse and treatment outcomes. Arch Gen Psychiatry. 2011;68:942–52. doi: 10.1001/archgenpsychiatry.2011.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SINHA R, TSOU K, GU P, GUARNACCIA J, FOX H, TUIT K. Alcohol effects on neuroendocrine and metabolic responses to stress and motivation for alcohol intake. Alcoholism: Clinical and Experimental Research. 2013 Sep;37(Suppl 2):287A. [Google Scholar]
- SINHA R, FUSE T, AUBIN LR, O’MALLEY SS. Psychological stress, drug-related cues and cocaine craving. Psychopharmacology (Berl) 2000;152:140–8. doi: 10.1007/s002130000499. [DOI] [PubMed] [Google Scholar]
- SINHA R, LOVALLO WR, PARSONS OA. Cardiovascular differentiation of emotions. Psychosom Med. 1992;54:422–35. doi: 10.1097/00006842-199207000-00005. [DOI] [PubMed] [Google Scholar]
- SINHA R, TALIH M, MALISON R, COONEY N, ANDERSON GM, KREEK MJ. Hypothalamic-pituitary-adrenal axis and sympatho-adreno-medullary responses during stress-induced and drug cue-induced cocaine craving states. Psychopharmacology (Berl) 2003;170:62–72. doi: 10.1007/s00213-003-1525-8. [DOI] [PubMed] [Google Scholar]
- SOBELL LC, SOBELL MB, LEO GI, CANCILLA A. Reliability of a timeline method: assessing normal drinkers’ reports of recent drinking and a comparative evaluation across several populations. Br J Addict. 1988;83:393–402. doi: 10.1111/j.1360-0443.1988.tb00485.x. [DOI] [PubMed] [Google Scholar]
- VOLPATO S, PAHOR M, FERRUCCI L, SIMONSICK EM, GURALNIK JM, KRITCHEVSKY SB, FELLIN R, HARRIS TB. Relationship of alcohol intake with inflammatory markers and plasminogen activator inhibitor-1 in well-functioning older adults: the Health, Aging, and Body Composition study. Circulation. 2004;109:607–12. doi: 10.1161/01.CIR.0000109503.13955.00. [DOI] [PubMed] [Google Scholar]
- YASUKAWA H, OHISHI M, MORI H, MURAKAMI M, CHINEN T, AKI D, HANADA T, TAKEDA K, AKIRA S, HOSHIJIMA M, HIRANO T, CHIEN KR, YOSHIMURA A. IL-6 induces an anti-inflammatory response in the absence of SOCS3 in macrophages. Nat Immunol. 2003;4:551–6. doi: 10.1038/ni938. [DOI] [PubMed] [Google Scholar]