Abstract
Purpose
Early research suggests prolonged ischemic time in older donor lungs is associated with decreased survival following lung transplantation. The purpose of this study was to determine if this association holds in the post-LAS era.
Methods
We analyzed the UNOS database 2005–2013 for adult recipients of cadaveric lung transplants. Cox proportional hazards modeling was utilized to determine the association of donor age, ischemic time, and the interaction of donor age and ischemic time with transplant-free survival.
Results
11,835 patients met criteria. Median donor age was 32 years and median ischemic time was 4.9hr. Cox modeling demonstrated that donor age 50–60 (adjusted HR: 1.11) and ≥60 (adjusted HR: 1.42) were associated with reduced overall survival. Neither ischemic time nor interaction of ischemic time and donor age were significantly associated with overall survival. Sub-analysis demonstrated that this finding held true for patients undergoing either single or bilateral lung transplantation.
Conclusions
Prolonged ischemic time is not associated with decreased overall survival in patients undergoing lung transplantation regardless of the donor’s age. However, donor age >50 is independently associated with decreased survival. The lack of an association between ischemic time and survival should encourage broader geographic allocation of pulmonary allografts.
Introduction
Lung transplantation is the gold-standard therapy for end-stage lung disease throughout the world, with 3,719 transplants reported to the International Society of Heart and Lung Transplantation (ISHLT) registry in 2012, a registry which captures roughly two-thirds of the world’s lung transplant activity.1 Unfortunately, despite increasing demand for lung transplantation in light of improving post-transplant survival and a growing population with end-stage lung disease, transplantation rates are plateauing, likely due to supply constraints.1,2 Some countries have turned to an “opt-out” organ donation approach, in which patients must consent to avoid organ donation, instead of consent to allow organ donation as is the process in the United States, which has led to an increase in organ transplantation in these countries.3 Others have begun to investigate alternative methods for increasing the donor pool, such as ex-vivo lung perfusion or enhanced utilization of extended criteria donors.4,5
In an attempt to better allocate donor organs as well as improve post-transplant survival, previous research has attempted to determine donor-related factors associated with outcomes following lung transplantation. Donor smoking status, donor arterial oxygenation, size mismatch, and donor cancer history have all been demonstrated to be associated with survival following lung transplantation.6–8 Initial work performed by Meyer and colleagues demonstrated that independently, donor age and ischemic time were not significantly associated with reduced survival, but when a prolonged ischemic time was combined with an older donor, this was associated with a significantly reduced survival.9,10 However, the majority of this work was performed prior to the development of current preservation techniques and the implementation of the Lung Allocation Score (LAS), both of which have dramatically transformed the process of organ allocation among lung transplant recipients.11,12 Due to these substantial changes, we sought to re-examine the association of donor age and ischemic time with long-term survival following lung transplantation in the modern (post-LAS) era.
Methods
United Network for Organ Sharing
The Standard Transplant Analysis and Research (STAR) dataset files from the United Network for Organ Sharing (UNOS) were utilized. These files contain data on all organ transplantations performed in the United States since October 1, 1987. These data are available to researchers from UNOS/Organ Procurement and Transplantation Network member institutions.13
Patient Population
Institutional review board approval was obtained prior to performing the analysis. STAR dataset files were queried for patients 18 years of age or older undergoing a cadaveric lung transplant between May 2005 (following the introduction of the LAS) and September 2013. Patients who received a multi-organ transplant, had a previous transplant, were in the intensive care unit prior to transplant, were supported by either ventilator or extracorporeal membrane oxygenation prior to transplant, or had an intra-aortic balloon pump at the time of transplant were excluded. Recipients receiving allografts from donors reported as donation after circulatory death were excluded. In addition, patients with an unknown ischemic time or unknown donor age were also excluded.
Variables
Patient and donor baseline characteristics, transplant characteristics, operative variables, and outcomes were compiled. Diagnoses were categorized into four groups including “obstructive lung disease,” “restrictive lung disease,” “cystic fibrosis/immunodeficiency” and “other.” Functional status as defined by Karnofsky score was grouped into “performing activities of daily living [ADL] with no assistance” (Karnofsky score 70–100), “performing ADLs with assistance” (Karnofsky score 50–60), or “disabled/hospitalized” (Karnofsky score 10–40). Linearity was evaluated for all continuous variables by plotting the variable against the log odds of one-year mortality. Variables that were not linear were categorized based on a subjective assessment of the plot of the variable and the log odds of one-year mortality. Variables were not transformed in order to aid in clinical utility. Survival was defined as the time from transplant to either death or retransplantation. Living recipients were censored at the time of last follow-up.
Statistical Analysis
Patients were initially grouped by donor age and compared for baseline and transplant-related characteristics. Continuous variables were compared using the Kruskal-Wallis test while categorical variables were compared using either Fisher’s exact test or the chi-square test as appropriate. Patients were also grouped by ischemic time and compared in a similar manner.
Kaplan-Meier analysis and Cox proportional hazards regression modeling were then utilized in order to determine the association of transplant-free survival with both ischemic time and donor age. Variables included in the Cox model were based on clinical relevance and included recipient and donor characteristics as well as center volume over the study period. Recipient characteristics included in the model were age, sex, diagnosis group, hypertension, diabetes, body mass index (BMI), pre-transplant creatinine, functional status, bilateral versus single lung transplantation, and ischemic time. Donor characteristics included age and a diagnosis of diabetes. An interaction term was initially included between donor age and ischemic time, but a decision was made a priori to remove the term from the final model if found to be non-significant. A sub-group analysis was also performed among single (SOLT) and bilateral (BOLT) orthotopic lung transplant recipients in order to determine if the association of donor age and ischemic time and transplant-free survival varied among these cohorts.
The proportional hazards assumption was tested for all Cox models. A p-value of 0.05 was used to define statistical significance. All statistical analyses were performed using R version 3.0.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
A total of 11,835 patients met study criteria, including 4,060 (34.3%) patients who underwent SOLT and 7,775 (65.7%) patients who underwent BOLT. The median donor age was 32 years (interquartile range [IQR]: 21, 46) while the median ischemic time was 4.9 hours (IQR: 3.9, 6.0), however there was substantial variation in both (Figure 1A & 1B).
Figure 1.
Variation in donor age (A) and ischemic time (B) among the study population.
Neither the association between both donor age and one-year survival nor ischemic time and one-year survival were found to be linear. Both predictor variables were therefore divided into categories based on an assessment of this association. Donor age was divided into ages 0–20 (n=2,439, 21.7%), 21–50 (n=6,725, 59.8%), 50–59 (n=1,625, 14.5%), and 60 or older (n=456, 4.1%). Recipients who received lungs from older donors tended to be older, were more likely to be female, were more likely to have obstructive lung disease, and had worse functional status (Table 1). Furthermore, patients who received lungs from older donors also tended to spend less time on the waiting list. Lastly, patients who received lungs from older donors tended to receive their transplant at higher-volume centers.
Table 1.
Baseline characteristics by donor age.
| Variable | Overall | Age 20 or Younger | Age 21–50 | Age 50–59 | Age 60 or Older | P-value |
|---|---|---|---|---|---|---|
| N | 11,835 | 2553 (21.6%) | 7072 (59.8%) | 1738 (14.7%) | 472 (4.0%) | |
| Recipient Characteristics | ||||||
| Age (years) | 59 (50, 64) | 58 (47, 64) | 58 (49, 64) | 60 (52, 65) | 62 (56, 65) | < 0.001 |
| Female | 4,818 (40.7%) | 1,008 (39.5%) | 2,810 (39.7%) | 769 (44.2%) | 231 (48.9%) | < 0.001 |
| Race/Ethnicity | 0.226 | |||||
| White/Caucasian | 9,937 (84.0%) | 2,151 (84.3%) | 5,931 (83.9%) | 1,437 (82.7%) | 418 (88.6%) | |
| Black/African-American | 1,017 (8.6%) | 218 (8.5%) | 619 (8.8%) | 153 (8.8%) | 27 (5.7%) | |
| Hispanic | 644 (5.4%) | 136 (5.3%) | 377 (5.3%) | 111 (6.4%) | 20 (4.2%) | |
| Other | 237 (2.0%) | 48 (1.9%) | 145 (2.1%) | 37 (2.1%) | 7 (1.5%) | |
| Etiology of Lung Disease | < 0.001 | |||||
| Obstructive Lung Disease | 4,028 (34.0%) | 821 (32.2%) | 2,402 (34.0%) | 608 (35.0%) | 197 (41.7%) | |
| Restrictive Lung Disease | 5,972 (50.5%) | 1,287 (50.4%) | 3,530 (49.9%) | 914 (52.6%) | 241 (51.1%) | |
| Cystic Fibrosis/Immunodeficiency | 1,455 (12.3%) | 361 (14.1%) | 906 (12.8%) | 167 (9.6%) | 21 (4.4%) | |
| Other | 380 (3.2%) | 84 (3.3%) | 234 (3.3%) | 49 (2.8%) | 13 (2.8%) | |
| Hypertension | 439 (3.7%) | 114 (4.5%) | 245 (3.5%) | 61 (3.5%) | 19 (4.0%) | 0.133 |
| Diabetes | 2,098 (17.9%) | 429 (16.9%) | 1,265 (18.0%) | 323 (18.7%) | 81 (17.3%) | 0.468 |
| Body Mass Index (kg/m2) | 25 (22, 29) | 25 (21, 28) | 25 (22, 29) | 25 (22, 28) | 26 (22, 28) | 0.001 |
| Steroid Treatment (Pre-Op) | 5,324 (46.5%) | 1,100 (44.5%) | 3,192 (46.7%) | 810 (48.0%) | 222 (48.2%) | 0.105 |
| Recipient Smoking History | 7,199 (60.8%) | 1,495 (58.6%) | 4,280 (60.5%) | 1,107 (63.7%) | 317 (67.2%) | < 0.001 |
| Creatinine at Time of Transplant | 0.8 (0.7, 1) | 1 (1, 1) | 1 (1, 1) | 1 (1, 1) | 1 (1, 1) | 0.540 |
| Functional Status at Transplant | 0.114 | |||||
| ADL With No Assistance | 3,541 (29.9%) | 793 (31.1%) | 2,098 (29.7%) | 528 (30.4%) | 122 (25.8%) | |
| ADL With Assistance | 4,774 (40.3%) | 1,046 (41.0%) | 2,856 (40.4%) | 687 (39.5%) | 185 (39.2%) | |
| Disabled/Hospitalized | 3,344 (28.3%) | 673 (26.4%) | 2,014 (28.5%) | 502 (28.9%) | 155 (32.8%) | |
| LAS at Match | 40 (35, 49) | 39 (35, 49) | 40 (35, 49) | 39 (35, 49) | 38 (34, 47) | 0.106 |
| O2 Requirement at Rest | 3 (2, 4) | 3 (2, 4) | 3 (2, 4) | 3 (2, 4) | 3 (2, 4) | 0.399 |
| Six Minute Walk Distance (ft) | 820 (459, 1,138) | 808 (449, 1,154) | 820 (467, 1,125) | 908 (500, 1,176) | 760 (458, 1,070) | 0.598 |
| Known History of Malignancy | 838 (7.1%) | 165 (6.5%) | 498 (7.1%) | 127 (7.3%) | 48 (10.2%) | 0.037 |
| Known to be Working at time of Transplant | 1,144 (10.0%) | 272 (11.1%) | 693 (10.2%) | 141 (8.4%) | 38 (8.5%) | 0.021 |
| Donor Characteristics | ||||||
| Donor PO2 on 100% O2 (mmHg) | 421 (242, 492) | 434 (228, 505) | 417 (238, 489) | 413 (275, 485) | 423 (286, 500) | 0.023 |
| Donor Diabetes | 804 (6.8%) | 27 (1.1%) | 406 (5.8%) | 281 (16.2%) | 90 (19.1%) | < 0.001 |
| Operative Characteristics | ||||||
| Bilateral Transplant | 7,775 (65.7%) | 1,697 (66.5%) | 4,625 (65.4%) | 1,159 (66.7%) | 294 (62.3%) | 0.246 |
| Ischemic Time (hours) | 5 (4, 6) | 5 (4, 6) | 5 (4, 6) | 5 (4, 6) | 5 (4, 6) | 0.175 |
| Time on Waiting List (days) | 72 (21, 226) | 82 (24, 236) | 70 (20, 220) | 70 (20, 230) | 69 (18, 243) | 0.077 |
| Center Volume (# of Cases in Study Period) | 577 (373, 874) | 542 (373, 829) | 574 (373, 874) | 653 (406, 1,201) | 752 (450, 1,201) | < 0.001 |
ADL – Activities of Daily Living. LAS – Lung Allocation Score. Continuous variables are presented as median (interquartile range) while categorical variables are presented as frequency (percentage).
Ischemic time was also divided into four groups; less than four hours (n=2,898, 24.8%), four to six hours (n=5,439, 48.4%), six to eight hours (n=2,372, 21.1%), and greater than eight hours (n=536, 4.8%). Patients who received lungs subjected to longer ischemic times tended to be younger, were less likely to be female, were less likely to have obstructive lung disease and were more likely to have cystic fibrosis, and had better pre-operative functional status (Table 2). Patients with longer ischemic times were also more likely to undergo BOLT versus SOLT, and were also more likely to be transplanted at higher volume centers.
Table 2.
Baseline characteristics by ischemic time.
| Variable | Overall | 4 Hours or Less | 4–6 Hours | 6–8 Hours | Greater than 8 Hours | P-value |
|---|---|---|---|---|---|---|
| N | 11,835 | 3001 (25.4%) | 5693 (48.1%) | 2563 (21.7%) | 578 (4.9%) | |
| Recipient Characteristics | ||||||
| Age (years) | 59 (50, 64) | 61 (54, 65) | 59 (50, 64) | 56 (44, 63) | 56 (44, 63) | < 0.001 |
| Female | 4,818 (40.7%) | 1,367 (45.6%) | 2,305 (40.5%) | 951 (37.1%) | 195 (33.7%) | < 0.001 |
| Race | 0.052 | |||||
| White/Caucasian | 9,937 (84.0%) | 2,484 (82.8%) | 4,783 (84.0%) | 2,175 (84.9%) | 495 (85.6%) | |
| Black/African-American | 1,017 (8.6%) | 272 (9.1%) | 465 (8.2%) | 231 (9.0%) | 49 (8.5%) | |
| Hispanic | 644 (5.4%) | 174 (5.8%) | 335 (5.9%) | 110 (4.3%) | 25 (4.3%) | |
| Other | 237 (2.0%) | 71 (2.4%) | 110 (1.9%) | 47 (1.8%) | 9 (1.6%) | |
| Etiology of Lung Disease | < 0.001 | |||||
| Obstructive Lung Disease | 4,028 (34.0%) | 1,203 (40.1%) | 1,874 (32.9%) | 766 (29.9%) | 185 (32.0%) | |
| Restrictive Lung Disease | 5,972 (50.5%) | 1,548 (51.6%) | 2,944 (51.7%) | 1,206 (47.1%) | 274 (47.4%) | |
| Cystic Fibrosis/Immunodeficiency | 1,455 (12.3%) | 181 (6.0%) | 678 (11.9%) | 493 (19.2%) | 103 (17.8%) | |
| Other | 380 (3.2%) | 69 (2.3%) | 197 (3.5%) | 98 (3.8%) | 16 (2.8%) | |
| Hypertension | 439 (3.7%) | 136 (4.5%) | 215 (3.8%) | 69 (2.7%) | 19 (3.3%) | 0.004 |
| Diabetes | 2,098 (17.9%) | 475 (16.0%) | 1,014 (17.9%) | 505 (19.8%) | 104 (18.2%) | 0.003 |
| Body Mass Index (kg/m2) | 25.2 (21.5, 28.6) | 26 (22, 29) | 25 (21, 28) | 25 (21, 28) | 25 (21, 27) | < 0.001 |
| Steroid Treatment (Pre-Op) | 5,324 (46.5%) | 1,408 (48.4%) | 2,601 (47.3%) | 1,062 (42.8%) | 253 (44.9%) | < 0.001 |
| Recipient Smoking History | 7,199 (60.8%) | 1,982 (66.0%) | 3,475 (61.0%) | 1,425 (55.6%) | 317 (54.8%) | < 0.001 |
| Creatinine at Time of Transplant | 0.8 (0.7, 1) | 1 (1, 1) | 1 (1, 1) | 1 (1, 1) | 1 (1, 1) | 0.373 |
| Functional Status at Transplant | < 0.001 | |||||
| ADL With No Assistance | 3,541 (29.9%) | 912 (30.4%) | 1,642 (28.8%) | 820 (32.0%) | 167 (28.9%) | |
| ADL With Assistance | 4,774 (40.3%) | 1,309 (43.6%) | 2,363 (41.5%) | 918 (35.8%) | 184 (31.8%) | |
| Disabled/Hospitalized | 3,344 (28.3%) | 735 (24.5%) | 1,597 (28.1%) | 789 (30.8%) | 223 (38.6%) | |
| LAS at Match | 39 (35, 49) | 38 (34, 46) | 40 (35, 49) | 41 (35, 51) | 41 (35, 51) | < 0.001 |
| O2 Requirement at Rest | 3 (2, 4) | 3 (2, 4) | 3 (2, 4) | 3 (2, 5) | 3 (2, 6) | < 0.001 |
| Six Minute Walk Distance (ft) | 820 (459, 1,138) | 770 (412, 1,078) | 828 (482, 1,120) | 896 (470, 1,200) | 900 (528, 1,192) | 0.064 |
| Known History of Malignancy | 838 (7.1%) | 233 (7.8%) | 367 (6.5%) | 188 (7.3%) | 50 (8.7%) | 0.046 |
| Known to be Working at time of Transplant | 1,144 (10.0%) | 311 (10.9%) | 554 (10.1%) | 237 (9.5%) | 42 (7.5%) | 0.070 |
| Donor Characteristics | ||||||
| Donor PO2 on 100% O2 (mmHg) | 421 (242, 492) | 407 (200, 485) | 423 (251, 494) | 427 (286, 498) | 431 (338, 499) | < 0.001 |
| Donor Age | 32 (21, 46) | 31 (21, 45) | 32 (21, 47) | 32 (22, 47) | 33 (22, 48) | 0.037 |
| Donor Diabetes | 804 (6.8%) | 193 (6.4%) | 386 (6.8%) | 181 (7.1%) | 44 (7.7%) | 0.658 |
| Transplant Characteristics | ||||||
| Bilateral Transplant | 7,775 (65.7%) | 1,150 (38.3%) | 3,810 (66.9%) | 2,292 (89.4%) | 523 (90.5%) | < 0.001 |
| Time on Waiting List (days) | 72 (21, 226) | 80 (25, 232) | 75 (22, 229) | 64 (19, 215) | 44 (11, 178) | 0.140 |
| Center Volume (# of Cases in Dataset) | 577 (373, 874) | 535 (335, 789) | 574 (387, 874) | 653 (393, 1,337) | 1,201 (419, 1,337) | < 0.001 |
ADL – Activities of Daily Living. LAS – Lung Allocation Score. Continuous variables are presented as median (interquartile range) while categorical variables are presented as frequency (percentage).
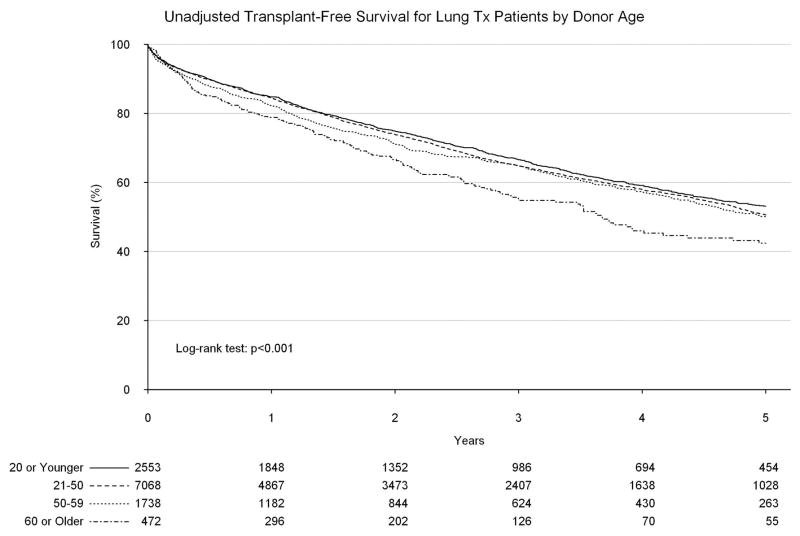
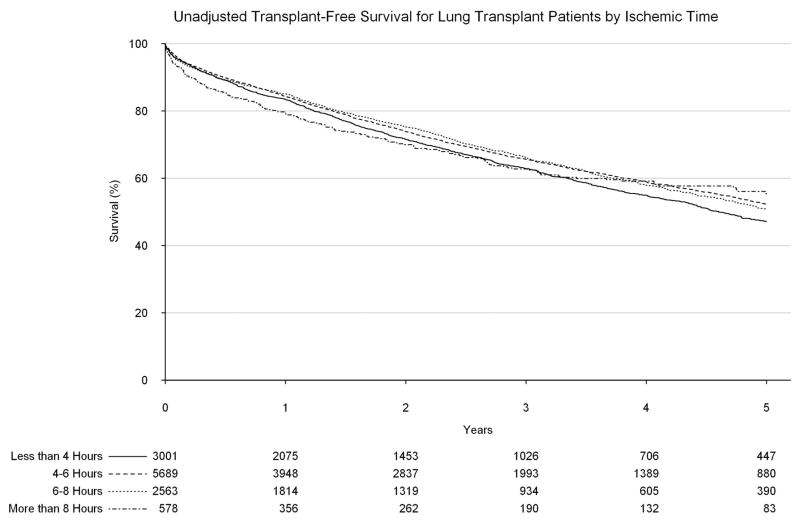
Upon unadjusted analysis, a donor age of 60 or greater was found to be associated with a significantly worse long-term survival as compared to younger donor ages, with a five-year survival of only 42.4% (95% confidence interval [CI]: 36.5%, 49.3%, Figure 2). An ischemic time of greater than 8 hours was similarly found to be associated with worse survival within 90 days of transplant (compared to less than four hours; hazard ratio [HR]: 1.48, 95% CI: 1.10, 1.98, Figure 3), however after 90 days, ischemic time of greater than 8 hours was associated with a significantly improved long-term survival, likely secondary to the high proportion of BOLTs found in this group (HR: 0.82, 95% CI: 0.69, 0.98).
Figure 2.
Transplant-free survival by donor age.
Figure 3.
Transplant-free survival by ischemic time.
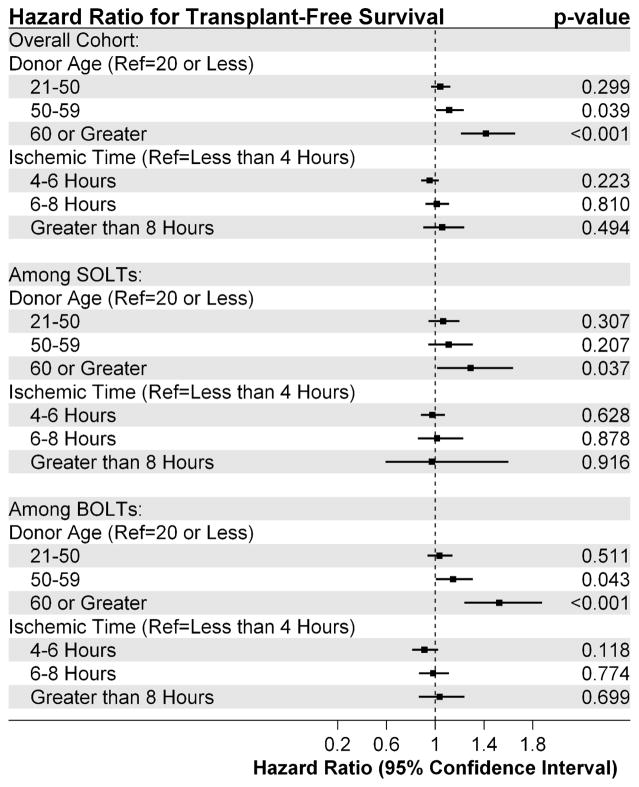
Upon adjustment with Cox modeling, the interaction term between donor age and ischemic time was consistently found to be non-significant, and was therefore removed from the model. In the overall cohort, after adjustment, only donor age greater than 50 remained significantly associated with reduced long-term survival (Donor Age 50–60: adjusted HR: 1.11, 95% CI: 1.01, 1.24, Donor Age ≥60: adjusted HR: 1.42, 95% CI: 1.21, 1.65, Figure 4). Ischemic time was not found to be significantly associated with long-term survival. Among patients undergoing a SOLT, donor age greater than 60 remained significantly associated with reduced long-term survival (adjusted HR: 1.29, 95% CI: 1.02, 1.64). Among patients undergoing a BOLT, donor age between 50 and 60 was associated with significantly reduced long-term survival (adjusted HR: 1.15, 95% CI: 1.00, 1.31) as was a donor age greater than 60 (adjusted HR: 1.52, 95% CI: 1.24, 1.88).
Figure 4.
Association between donor age and ischemic time and transplant-free survival among the overall cohort, as well as among patients receiving single (SOLT) and bilateral orthotopic lung transplants (BOLT).
Discussion
The last decade has seen substantial modifications in lung transplantation, much of it secondary to the implementation of the LAS in 2005.12 Furthermore, post-transplant survival has been steadily improving over time, although this is less likely to be associated with the LAS and more likely secondary to improving post-transplant care as well as more modern transplantation techniques.2,14 In this study, we have demonstrated that in the current era, there does not appear to be an interaction between donor age and ischemic time as seen previously by Meyer and colleagues.9,10 We did demonstrate, however, that donor age continues to be a significant factor in long-term survival, especially among donors greater than 50 years of age.
We are not the first to demonstrate an association between advanced donor age and reduced survival among lung transplant recipients. Pilcher and colleagues demonstrated that donor age as a continuous variable was associated with early graft dysfunction following transplantation as defined by PAO2/FIO2 ratios (arterial blood gas partial pressure of oxygen/fraction of inspired oxygen), although not all studies agreed with these findings.8,15 Other studies have demonstrated that the association between donor age and survival may be dependent on other factors such as recipient pulmonary hypertension status or prolonged cardiopulmonary bypass times.16 More recent analyses by Hayes and colleagues demonstrated that this association may be dependent on recipient age as well, as older donor lungs only appeared to have a significant negative association with long-term survival among older recipients.17 Lastly Bittle and colleagues demonstrated that the use of donor lungs greater than 65 years of age was associated with significantly reduced survival.18
Although Meyer and colleagues did not demonstrate an “independent” association between ischemic time and survival, a study performed by Thabut and colleagues between 1987 and 1998 did demonstrate an association between ischemic time and both early graft function as well as long-term survival.9,10,19 In our examination of the more current experience, we were not able to demonstrate either an association between ischemic time and overall survival, nor an interaction between ischemic time and donor age and overall survival. There are likely several reasons for the different conclusions, most importantly due to improvements in organ preservation and other transplantation techniques. An important concern with our findings was our inability to demonstrate an association between ischemic time and overall survival, a potential type II statistical error. This is unlikely, as a key change in the modern era has been the use of longer ischemic times in general, which contributed to the larger number of patients in the prolonged ischemia group in our study (536 as compared to 384 in the Novick study and 245 in the Meyer study).9,10 Therefore it is very unlikely that our findings are solely due to insufficient power. As a final note with regards to early and late survival in recipients of allografts with long ischemic times, it appears that an early survival disadvantage is tempered by a modest survival advantage late (after 90 days). This finding may be related to an increased fraction of BOLT recipients receiving grafts with longer ischemic times, in which increased early morbidity and mortality related to the more invasive procedure is tempered by a late survival advantage. These data should be considered in the subsequent analysis in which the role of ischemic time is examined separately within BOLT and SOLT recipients.
Trends in the use of what many may consider to be “extended criteria organs” is also worthy of consideration, as certain variables tend to correlate with the use of lungs from older donors and with longer ischemic times. Older patients with obstructive lung diseases tend to be much more likely to receive lungs from older donors than younger patients with cystic fibrosis, likely due to an intentional attempt to match younger donors with younger recipients. This would also explain the trends seen by BMI and smoking history. Interestingly, these trends reversed when investigating ischemic time, in that lungs with longer ischemic times tend to be used in younger patients who are less likely to have obstructive lung disease and more likely to have diagnoses such as cystic fibrosis, although this may be related to the higher use of bilateral lung transplantation among younger patients with cystic fibrosis. There was no significant difference with regards to either donor age or ischemic time and waiting list time, demonstrating that these factors may not be playing a role in organ allocation at this time.
We also demonstrated that higher volume centers tended to be more likely to transplant more “marginal” donors, both with regards to using lungs from older donors, as well as using lungs which required longer periods of ischemia. Transplantation at a higher-volume center has previously been associated with improved survival, however in order to attain this volume, these centers are likely more capable of accepting organs from extended criteria donors or organs from greater distances away.20 Nonetheless, we included center volume in our model, and therefore it is unlikely that the optimal survival associated with a high center volume would have masked the association between either of the primary predictors and long-term survival.
There are also limitations to this study which limit the generalizability of our findings. First, although there is substantial variation in both donor age and ischemic time in the UNOS database, there are few transplants performed at the high-end of the spectrum for both, thus limiting the power of this study. It is obvious that there is a time point at which lungs not being perfused will not perform as well following transplant, however as centers currently limit ischemic time as much as possible, we did not have the power to determine this point. Second, as a retrospective study of a national registry, there is always the potential for unmeasured confounding which could not be accounted for in the adjustment, including organ preservation methods and variation in post-transplant practices. Third, we decided to categorize the primary predictors (donor age and ischemic time) instead of transforming the variables as continuous variables, which may also have reduced the power of the study. However, categorized variables are much more useful clinically, which is the reason we pursued this approach.
In conclusion, we have demonstrated that in contrast to previous studies, prolonged ischemic time is not significantly associated with overall survival in patients undergoing either a SOLT or BOLT regardless of the donor’s age. Although we cannot determine from this analysis the etiology for this change from previous studies, we hypothesize that it may also be secondary to changes in practice and organ allocation following the implementation of the LAS, or related to improved preservation techniques and post-transplant care. In addition, we have continued to demonstrate that advanced donor age is associated with decreased long-term survival, although the donor age at which survival begins to decline varies by the type of transplant. In appropriately-selected recipients and in centers with high volume, we suggest that the lack of an association between ischemic time and survival should encourage a cautiously increased geographic allocation of pulmonary allografts.
Acknowledgments
Funding: Institutional Funding was the primary funding source for this study. In addition, this work was supported by the NIH funded Cardiothoracic Surgery Trials Network (B.C.G., B.R.E., and M.G.H.), 5U01HL088953-05.
The data reported here have been supplied by the United Network for Organ Sharing as the contractor for the Organ Procurement and Transplantation Network. The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the OPTN or the U.S. Government. In addition, this work was supported by the NIH funded Cardiothoracic Surgery Trials Network (B.C.G., B. R. E., and M.G.H), 5U01HL088953-05.
Footnotes
Conflicts of Interest: The co-authors have no conflicts of interest to report.
Conflicts of Interest
The authors have no financial conflicts of interest to report.
References
- 1.Yusen RD, Edwards LB, Kucheryavaya AY, et al. The registry of the International Society for Heart and Lung Transplantation: thirty-first adult lung and heart-lung transplant report--2014; focus theme: retransplantation. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2014;33(10):1009–1024. doi: 10.1016/j.healun.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 2.Christie JD, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for Heart and Lung Transplantation: 29th adult lung and heart-lung transplant report—2012. The Journal of Heart and Lung Transplantation. 2012;31(10):1073–1086. doi: 10.1016/j.healun.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 3.Shepherd L, O’Carroll RE, Ferguson E. An international comparison of deceased and living organ donation/transplant rates in opt-in and opt-out systems: a panel study. BMC medicine. 2014;12:131. doi: 10.1186/s12916-014-0131-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ingemansson R, Eyjolfsson A, Mared L, et al. Clinical transplantation of initially rejected donor lungs after reconditioning ex vivo. The Annals of thoracic surgery. 2009;87(1):255–260. doi: 10.1016/j.athoracsur.2008.09.049. [DOI] [PubMed] [Google Scholar]
- 5.Pêgo-Fernandes P, Samano M, Fiorelli A, et al. Recommendations for the use of extended criteria donors in lung transplantation. Paper presented at: Transplantation proceedings; 2011. [DOI] [PubMed] [Google Scholar]
- 6.Bonser RS, Taylor R, Collett D, Thomas HL, Dark JH, Neuberger J. Effect of donor smoking on survival after lung transplantation: a cohort study of a prospective registry. Lancet. 2012;380(9843):747–755. doi: 10.1016/S0140-6736(12)60160-3. [DOI] [PubMed] [Google Scholar]
- 7.Orens JB, Boehler A, De Perrot M, et al. A review of lung transplant donor acceptability criteria. The Journal of heart and lung transplantation. 2003;22(11):1183–1200. doi: 10.1016/s1053-2498(03)00096-2. [DOI] [PubMed] [Google Scholar]
- 8.Pilcher DV, Snell GI, Scheinkestel CD, Bailey MJ, Williams TJ. High donor age, low donor oxygenation, and high recipient inotrope requirements predict early graft dysfunction in lung transplant recipients. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2005;24(11):1814–1820. doi: 10.1016/j.healun.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 9.Meyer DM, Bennett LE, Novick RJ, Hosenpud JD. Effect of donor age and ischemic time on intermediate survival and morbidity after lung transplantation. Chest. 2000;118(5):1255–1262. doi: 10.1378/chest.118.5.1255. [DOI] [PubMed] [Google Scholar]
- 10.Novick RJ, Bennett LE, Meyer DM, Hosenpud JD. Influence of graft ischemic time and donor age on survival after lung transplantation. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 1999;18(5):425–431. doi: 10.1016/s1053-2498(98)00057-6. [DOI] [PubMed] [Google Scholar]
- 11.Kozower BD, Meyers BF, Smith MA, et al. The impact of the lung allocation score on short-term transplantation outcomes: a multicenter study. The Journal of thoracic and cardiovascular surgery. 2008;135(1):166–171. doi: 10.1016/j.jtcvs.2007.08.044. [DOI] [PubMed] [Google Scholar]
- 12.Lingaraju R, Blumenthal NP, Kotloff RM, et al. Effects of lung allocation score on waiting list rankings and transplant procedures. The Journal of heart and lung transplantation. 2006;25(9):1167–1170. doi: 10.1016/j.healun.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 13.Organ Procurement and Transplantation Network, U.S. Department of Health & Human Services. [Accessed: March 31, 2015]; http://optn.transplant.hrsa.gov/data/request_main.asp?refer=true.
- 14.Merlo CA, Weiss ES, Orens JB, et al. Impact of US Lung Allocation Score on survival after lung transplantation. The Journal of Heart and Lung Transplantation. 2009;28(8):769–775. doi: 10.1016/j.healun.2009.04.024. [DOI] [PubMed] [Google Scholar]
- 15.Baldwin MR, Peterson ER, Easthausen I, et al. Donor age and early graft failure after lung transplantation: a cohort study. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2013;13(10):2685–2695. doi: 10.1111/ajt.12428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shigemura N, Horai T, Bhama JK, et al. Lung transplantation with lungs from older donors: recipient and surgical factors affect outcomes. Transplantation. 2014;98(8):903–908. doi: 10.1097/TP.0000000000000134. [DOI] [PubMed] [Google Scholar]
- 17.Hayes D, Jr, Black SM, Tobias JD, Higgins RS, Whitson BA. Influence of donor and recipient age in lung transplantation. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2015;34(1):43–49. doi: 10.1016/j.healun.2014.08.017. [DOI] [PubMed] [Google Scholar]
- 18.Bittle GJ, Sanchez PG, Kon ZN, et al. The use of lung donors older than 55 years: a review of the United Network of Organ Sharing database. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2013;32(8):760–768. doi: 10.1016/j.healun.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 19.Thabut G, Mal H, Cerrina J, et al. Graft ischemic time and outcome of lung transplantation: a multicenter analysis. American journal of respiratory and critical care medicine. 2005;171(7):786–791. doi: 10.1164/rccm.200409-1248OC. [DOI] [PubMed] [Google Scholar]
- 20.Thabut G, Christie JD, Kremers WK, Fournier M, Halpern SD. Survival differences following lung transplantation among US transplant centers. JAMA : the journal of the American Medical Association. 2010;304(1):53–60. doi: 10.1001/jama.2010.885. [DOI] [PubMed] [Google Scholar]