Figure 7. BEZ235 augments carboplatin and topotecan induced apoptosis of retinoblastoma tumor cells in vivo.
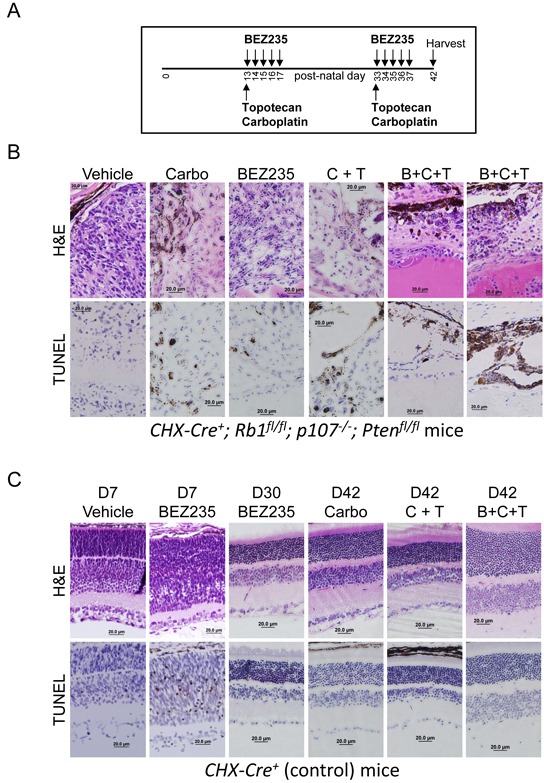
A. BEZ235 (50 μg/kg) was delivered by oral gavage on post-natal days 13 through 17, and 33 through 37. Topotecan (0.7 mg/kg) was delivered intraperitoneally on days 13 and 33. Carboplatin (100 μg/eye) was injected into the subconjunctival space on days 13 and 33. Mice were harvested at post-natal day 42 for analysis. B. CHX-Cre+; Rb1fl/fl; p107−/−; Ptenfl/fl pups were treated with the noted therapy as described by the schema in A. Eyes were harvested at day 42, fixed and analyzed for H&E and immunohistochemistry for TUNEL to detect apoptotic cells. Carbo indicates monotherapy with carboplatin. C + T is combined treatment of carboplatin and topotecan. B + C + T is the combined treatment of all three, and results depict eyes from at least 3 independent mice per group. C. CHX-Cre+ (control) pups were treated with the noted therapies. Vehicle or BEZ235 was administered daily to control mice beginning at post-natal day 2 and ending at day 7 when eyes were harvested for histological analysis (D7) to assess BEZ235-mediated apoptosis in the developing retina. BEZ235 was also given daily to control mice with mature retina beginning at day 25 until day 29 and eyes harvested at day 30 and analyzed for H&E and TUNEL to detect apoptotic cells (D30 BEZ235). The other listed therapies (Carbo, C+T, and B+C+T) were given to control mice as outlined by the schema in A and harvested for histology at day 42 from a minimum of three independent mice. Eyes were removed, fixed, and analyzed for H&E and TUNEL to detect apoptotic cells.
