Figure 5. Rad54 Phosphorylation during S Phase Causes Rad51 Removal from Stalled Replication Forks.
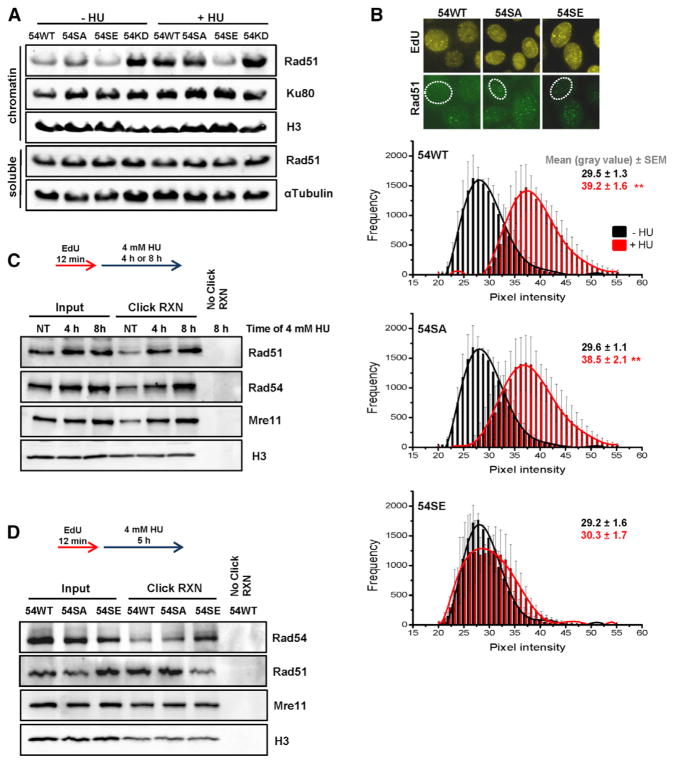
(A) Chromatin fraction of Rad51 in Rad54 mutants. HeLa clones were treated with siRad54 prior to HU treatment (4 mM for 5 hr), and chromatin fractions were analyzed by immunoblotting. The soluble fractions served as controls.
(B) Chromatin-bound Rad51 in Rad54 mutants. HeLa clones were treated with siRad54, co-treated with HU (0.5 mM for 2 hr) and EdU, and chromatin-bound Rad51 levels were analyzed by IF microscopy in EdU-positive nuclei. Rad51 showed a distribution of intensities with signals in the gray value range between 20 and 55 representing non-foci signals and intensities between 150 and 250 representing foci signals. The analysis was restricted to signals between 20 and 55. The mean ± SEM for each intensity is shown (n = 4).
(C) Analysis of proteins bound to stalled replication forks using iPOND. Hek293 cells were labeled with EdU, followed by different times of HU treatment. H3 signals were used to control the pull-down efficiency of EdU-labeled chromatin.
(D) Analysis of proteins bound to stalled replication forks in Rad54 mutants using iPOND. HeLa clones were treated with siRad54 prior to EdU labeling and HU treatment. H3 signals were used to control the pull-down efficiency of EdU-labeled chromatin.