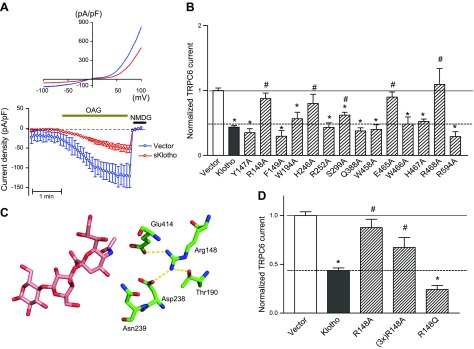
Figure 5.
A) sKlotho partially inhibits TRPC6 channel. Cultured HEK cells were cotransfected with plasmid encoding for TRPC6 and with or without sKlotho. Whole-cell recording of OAG-stimulated TRPC6 currents was performed 48 h later. OAG is membrane-permeant DAG analog. Top, current–voltage relationship curve of TRPC6 current revealing characteristic doubly-rectifying current. Bottom, time course of inward TRPC6 current density (current at −100 mV divided by capacity). At end of recording, N-methyl-d-glucamine was added to inhibit inward current in order to verify TRPC6-specific current. B) Comparison of activity of mutated sKlotho proteins compared to wild-type sKlotho activity. HEK cells were cotransfected with plasmid encoding for TRPC6 and for wild-type (Klotho) or mutant sKlotho (labeled for amino acid mutated, such as Y149A) or control empty vector (vector). Current density for vector transfected is 1. Current density for wild-type and mutant sKlotho is normalized to vector group; n = 6–15 each. *P < 0.01 vs. vector; #P < 0.05 vs. wild-type sKlotho. C) Illustration of hydrogen-bonding network formed by Arg148, showing why mutation of Arg148 can cause loss of binding. D) Effect of 3-fold excess of R148A mutant and R148Q mutant on TRPC6. Experimental paradigm as in A and B.