Abstract
Serralysin-like proteases are found in a wide variety of bacteria. These metalloproteases are frequently implicated in virulence and are members of the widely conserved RTX-toxin family. We identified a serralysin-like protease in the genome of a clinical isolate of S. marcescens that is highly similar to the canonical serralysin protein, PrtS. This gene was named serralysin-like protease E, SlpE, and was found in the majority (67%) of tested clinical isolates, but was absent from most tested non-clinical isolates including the insect pathogen and reference S. marcescens strain Db11. Purified recombinant SlpE exhibited calcium-dependent protease activity similar to metalloproteases PrtS and SlpB. Induction of slpE in the low-protease-producing S. marcescens strain PIC3611 highly elevated extracellular protease activity, and extracellular secretion required the lipD type 1 secretion system gene. Transcription of slpE was highly reduced in an eepR transcription factor mutant. Mutation of the slpE gene in a highly proteolytic clinical isolate reduced its protease activity, and evidence suggests that SlpE confers cytotoxicity of S. marcescens to the A549 airway carcinoma cell line. Together, these data reveal SlpE to be an EepR-regulated cytotoxic metalloprotease associated with clinical isolates of an important opportunistic pathogen.
Keywords: Metalloprotease, Enterobacteriaceae, Virulence factor, Cytotoxicity, Airway cells
1. Introduction
The serralysin protein, PrtS, is a cytotoxic virulence factor secreted by Serratia marcescens, an opportunistic bacterial pathogen. Serralysin-like proteases are zinc-dependent metalloproteases found in a wide group of bacteria [29]. These metalloproteases are implicated in virulence and have the widely conserved repeats-in-toxin (RTX) domain at their C-terminus [10], [17], [21], [29]. RTX domains have aspartate- and glycine-rich repeats that coordinate binding of calcium (Ca2+) which is necessary for proper folding of the protein [33]. Serralysin family proteins, including AprA from Pseudomonas aeruginosa, PrtS and SlpB from S. marcescens, PrtC from Erwinia chrysanthemi and many others, are secreted through type I secretion systems [5], [10]. In the case of S. marcescens, the secretion system for PrtS is composed of the LipB, LipC and LipD proteins [1], [19].
A few species of bacteria have several metalloprotease genes in their genomes [12], [22], although the reason for multiple serralysin family metalloproteases is unclear, given that these proteins generally have broad substrate specificity and maintain functionality over a reasonably broad range of temperature and pH [15], [33]. There are four predicted metalloprotease genes in the S. marcescens reference strain Db11 genome [27]. These included serralysin (prtS) and genes predicted to code for serralysin-like proteins, slpB, slpC, and slpD. Of these, only prtS and slpB produced extracellular metalloprotease activity when the genes were induced [27].
Previously, a protease was cloned from S. marcescens strain SM6 that was cytotoxic to HeLa cells in vitro when expressed from E. coli [6], [20]. In this study, we identified a protease with identical amino acid sequence to the SM6 protease in a clinical isolate of S. marcescens. We propose to rename the SM6 protease SlpE, because it is found in strains beyond SM6, and to follow the serralysin-like-protease nomenclature [27]. The purpose of this study was to expand upon previous studies [6] [20] by using defined mutations in clinical isolates, determining whether slpE was found in genomes of clinical isolates of S. marcescens, assessing SlpE cytotoxicity to a human airway cell line relative to PrtS and SlpB, testing whether the slpE was regulated by the EepR transcription factor and determining the secretion system required to secrete SlpE.
2. Materials and methods
2.1. Microorganisms and growth conditions
S. marcescesns isolates and E. coli were grown in lysogeny broth [3] with aeration or on agar plates. Antibiotics were used for conjugations to remove donor strains (tetracycline at 10 μg/ml) or to select for plasmids (kanamycin at 50 μg/ml and gentamicin at 10 μg/ml). Saccharomyces cerevisiae strain InvSc1 (Invitrogen) was grown in yeast extract peptone dextrose broth or on synthetic complete medium without uracil to select for plasmids. Microorganisms were grown at 30˚C. Pigmented environmental isolate of S. marcescens HQS was obtained from a soil sample on LB agar plates supplemented with tetracyline and confirmed as S. marcescens by PCR and phenotypic analysis. S. marcescens strain TM was isolated from the gut flora of the subterranean termite Reticulitermes sp. using 2.5% yeast extract agar plates. The 16S rDNA of the isolate is 99% identical to S. marcescens. The genome of strain TM was sequenced and assembled by Molecular Research DNA (MR DNA, Shallowater, TX, USA) and the genome was annotated and deposited in NCBI Genbank (accession number KT901292.1). Keratitis isolates were obtained from Regis Kowalski at the Charles T. Campbell Laboratory of Ophthalmic Microbiology. Other S. marcescens strains are cited later in the text or listed in Table 1.
Table 1. S. marcescens.
strains and plasmids
| Strain | Description | Reference or source |
|---|---|---|
| PIC3611 | Serratia marcescens wild type, PIC strain number 3611 | Presque Isle Cultures |
| K904 | Contact-lens-associated keratitis isolate | [18] |
| CMS2853 | K904 with prtS deletion mutation, ΔprtS | [27] |
| CMS4063 | PIC3611 lipD∷tn | [27] |
| CMS4098 | K904 ΔprtS ΔslpB | [27] |
| CMS4308 | K904 slpE∷pMQ543 | This study |
| CMS4309 | CMS4098 with slpE∷pMQ543 “triple mutant” | This study |
| pMQ118 | nptII, rpsL, oriT, oriR6K, URA3, CEN6/ARSH4 | [25] |
| pMQ125 | orip15a, PBAD-lacZa, oripRO1600, oriT, URA3, CEN6/ARSH4 | [25] |
| pMQ516 | pMQ125 + His7-slpE | This study |
| pMQ543 | pMQ118 + slpE internal fragment | This study |
2.2. DNA analysis
K904 genomic DNA was prepared using a commercial kit (5-PRIME AchievePure DNA cell and tissue), and was sequenced by ACGT Incorporated using Illumina technology to an average coverage of 113x. The contig containing slpE was submitted to GenBank (accession number KT901292), and the remaining genomic DNA will be reported in a separate study.
To test various isolates for the slpE gene, total DNA was isolated using Quick Extract DNA extraction solution (Epicentre). Primers for the conserved oxyR gene were used as a control to ensure the quality of the DNA preparation, and all samples were positive for the oxyR amplicon; these were (5′ to 3′) GGAGGGAAACAATGAATATTCG and GCTGTCGAGTTGCGCCAGC. Two primer sets were used to amplify slpE, and if either amplicon was productive, then the strain was considered positive for slpE. A subset of slpE amplicons was sequenced, verifying that the amplicon was slpE. Two primer sets were used to amplify slpE. As a first pass, a primer set that amplified an internal amplicon was used (5′ to 3′) CAAAGATGCGACGGTGACCTATTCC and CTAAACTGCCGGGTATCTTCG. If this set was negative for any given bacterial genome, a second set that amplified the entire slpE ORF was used (5′ to 3′) TTATTAAACGATAAAATCAACAGCGAC and GGTAATACCATGAGTGGAAACAAAAC. Amplicons were separated on agarose gels and the presence of a slpE band of the correct size with either primer set classified the strain as being positive for slpE.
2.3. Cloning and mutagenesis
Plasmid pMQ516 was generated to induce expression of slpE. The slpE open reading frame (ORF) was cloned with an N-terminal His7-tag under control of the PBAD promoter on vector pMQ125 using in vivo cloning with S. cerevisiae [24,25]. Plasmids are listed in Table 1.
The slpE gene was mutated in strain K904 and K904 ΔprtS ΔslpB by insertion of plasmid pMQ543. To make pMQ543, an internal fragment of slpE was amplified and cloned into plasmid pMQ118 that is unable to replicate in S. marcescens [25]. Mutants were generated by conjugation with E. coli bearing pMQ543 and selecting for kanamycin resistance. Kanamycin-resistant transformants were verified for the slpE∷pMQ543 mutation using PCR and maintained on kanamycin.
2.4. Cytotoxicity assay
Cytotoxicity was measured as previously described using Presto Blue (Life Technologies) [27]. Briefly, monolayers of human airway carcinoma cell line A549 [13] were challenged with filtered (0.22 μm) bacterial growth medium from cultures grown for 20 h and adjusted to OD600=2. The cell layers were incubated with supernatants for 4 h at 37˚C in a CO2 (5%) incubator. The media above the cells was replaced with fresh medium and incubated with Presto Blue for 1 h and fluorescence was measured using a plate reader (Biotek Synergy 2). A549 cells were grown in Dulbecco’s modified Eagle’s medium supplemented with fetal bovine serum (10%). The detergent Triton X-100 (0.25%) was used to lyse A549 cells to determine maximum cytotoxicity, and LB medium was used as a control to determine the absence of cytotoxicity.
2.5. SlpE purification and analysis
The pMQ516 plasmid with an N-terminal His7-tagged slpE ORF was used to transform E. coli EC100D pir-116 cells by electroporation. The resulting cells were grown overnight in the presence of gentamicin and used as an inoculum for 1 L expression cultures. Expression cultures were grown in LB supplemented with gentamicin. Arabinose (0.2% w/v) was added to the cells at OD600 ~0.6 to induce slpE expression and incubated at 37°C for 4–6 h. The SlpE protein was purified from inclusion bodies under denaturing conditions following protocols previously described for PrtS and AprA [8], [32]. Proteases were refolded by rapid dilution into PBS or PBS supplemented with 2 mM Ca2+ and incubated on ice for 15 min prior to analysis. Protease activity was assessed using a fluorescent casein substrate (EnzChek, Invitrogen) or by colorimetric substrate azocasein (Sigma) [8], [9], [26], [32]. The protease activities were assessed in 96-well plate format on a BioTek Synergy 4 multi-mode plate reader. Cleavage of either substrate induces change in fluorescence, EnzChek, or absorbance, azocasein, that was monitored as kinetic or endpoint measurements. For in vitro cleavage reactions, the activity of the refolded protease was assessed at 37° C after dilution to 1–5 uM final concentration. Mean enzymatic velocity was fit under steady-state conditions and normalized to wild type controls. Reactions lacking the proteases were included as controls for basal, background substrate cleavage [8],[32].
2.6. Serralysin modeling
The serralysin proteases (SlpB, C, D, E) were modeled using the Swiss Model server [4]. Protease sequences were first aligned using the T-coffee multiple sequence alignment tools and used to model the proteases using the 1.1Å structure S. marsescens serralysin, PDB code 5D7W. Homology models were subsequently refined using the Chiron server to minimize steric clashes [23].
2.7. Transcriptional analysis
RNA extraction from OD600 = 3.0 cultures and quantitative real-time PCR (qRT-PCR) analysis using the comparative CT method were performed as previously described [28]. Primers to detect slpE transcript were (5′ to 3′) ATTCTGATGGGCAAAGTTGG and TAAACTGCCGGGTATCTTCG. Primers to amplify the 16S rRNA gene as an internal control were previously described [28].
2.8. Statistical analysis
Two-tailed Fisher’s Exact test and one-way analysis of variance (ANOVA) with Tukey’s posttest were performed using Graphpad Prism software. Statistical significance was set p<0.05.
3. Results
3.1. An open reading frame for a serralysin-like protein identified in the genome of clinical S. marcescens isolates
An uncharacterized open reading frame was found during partial sequencing of the genome of S. marcescens K904, which is an ocular clinical isolate (GenBank accession KT901292.1). This open reading frame is predicted to code for a protein with an amino acid sequence 61% identical to the well-known virulence factor serralysin (PrtS), a metalloprotease. Interestingly, the candidate serralysin protein is absent from the genome of the sequenced reference strain Db11 that was originally isolated from Drosophila [16]. The candidate protease is here noted as serralysin-like protease E (SlpE). The predicted SlpE protein is 479 amino acids in length, compared to 504 for PrtS. It follows the same domain structure with a C-terminal predicted Ca2+ binding domain with repeating glycine/aspartate rich repeats and an N-terminal zinc-dependent protease domain (Fig. 1A) suggestive of a serralysin-family protease. BLASTP [2] queries indicated that a protein with the identical amino acid sequence, the metalloprotease gene of S. marcescens strain SM6, has been cloned and reported by Braunagel and Benedik [6].
Fig. 1. Analysis of S. marcescens serralysin family proteases.
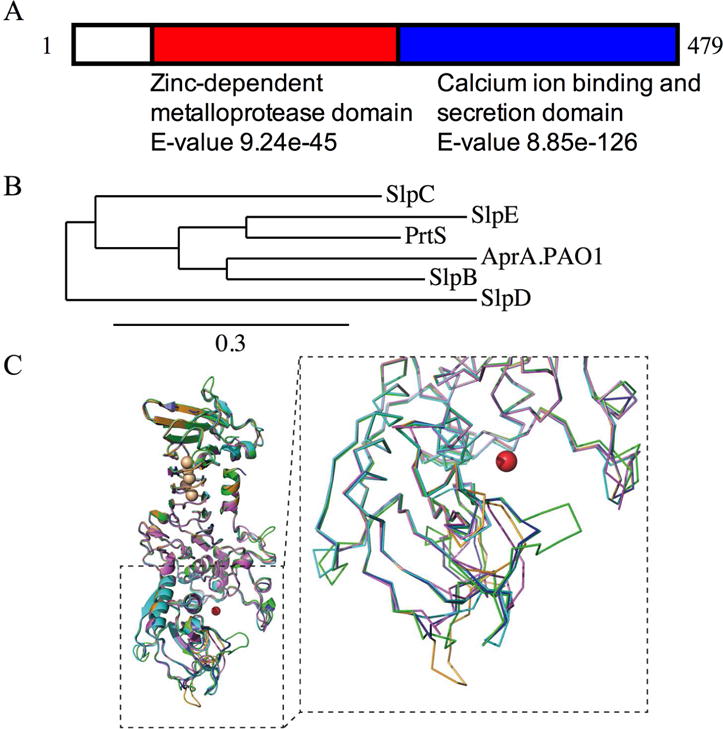
A. Schematic diagram of SlpE with predicted domains, determined by BLASTP. B. Phylogentic comparison of amino acid sequences of S. marcescens serralysin family proteases and AprA from P. aeruginosa strain PAO1. Tree made using one-click mode tree rendering program from www.Phylogeny.fr with default settings. The substitution numbers per site are proportional to the branch length. C. Homology models of the PrtB, C, D and E proteases are shown superposed onto the solved structure of serralysin (PrtS) (PDB code 5D7W). The serralysin structure is shown in blue aligned with SlpB (magenta), SlpC, (cyan), SlpD (green) and SlpE (orange). A close-up view of the protease domain is shown as a ribbon trace to highlight the local differences of the proteases near the active site. The dashed line indicates the region enlarged. The zinc co-factor, shown in the active site, is shown as a red sphere.
In strain K904, the slpE gene is upstream from, and in a predicted operon with, a predicted protease inhibitor gene. The slpE gene is 920 base pairs downstream from a divergently expressed gene predicted to code for a diguanylate cyclase as predicted by BLASTP.
Comparative sequence analysis of the recently described SlpB cytotoxic metalloprotease indicated that SlpB was closer in sequence to AprA from Pseudomonas aeruginosa than PrtS from S. marcescens, suggestive of horizontal gene transfer [27]. By contrast, SlpE, is most closely related to PrtS (Fig. 1B). Interestingly, whereas the multiple serralysin-family genes of E. chrysanthemi are grouped together on the chromosome [31], the serralysin genes from S. marcescens are not.
The serralysin proteases all share a common two-domain fold with an N-terminal protease domain and a C-terminal RTX domain. Given the high sequence identity and similarity across the serralysin family, homology models of the SlpB, C, D, and E proteases were generated. As expected, the serralysin protease structures are highly similar, with only minor changes in and around the active site (Fig. 1C). The Ca2+ and Zn2+ cofactor sites are conserved across the family and in all the structures analyzed to date. Small local changes surrounding the active site are seen in the various proteases and may account for changes in enzyme kinetics and substrate preference.
Because the slpE gene was absent in the Db11 genome, PCR analysis was used to test the prevalence of the slpE gene in clinical, environmental and laboratory strains of unknown origin of S. marcescens. However, it is likely that the laboratory strains are not clinical isolates because they are pigmented, and the vast majority of clinical isolates are non-pigmented [14]. In total, 25 out of 42 strains (60%) were positive for slpE amplicons. Of the clinical isolates, 24 of 36 were positive for slpE (67%), whereas only 1 of the 6 non-clinical isolates (17%) produced the slpE amplicon (p=0.032, Fisher’s exact test). A sample gel is shown in Fig. 2; gels for other strains are not shown. Five of the slpE amplicons were tested by sequencing and all were slpE DNA, indicating that the primers are specific.
Fig. 2. PCR analysis of S. marcescens genomes for the slpE gene.
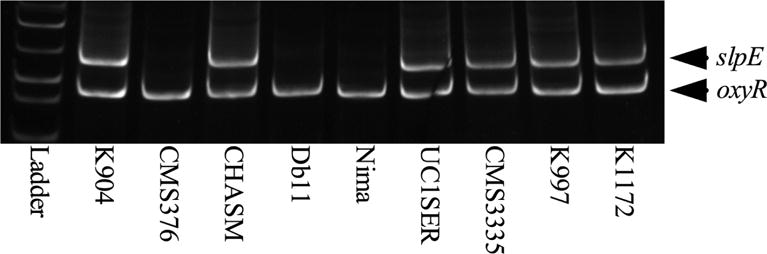
Chromosomal DNA from various S. marcescens strains was used in PCR reactions to identify an internal amplicon of the slpE gene. Amplification of an internal portion of the oxyR gene was used as a control for chromosomal DNA quality. Six of the nine strains shown here exhibited a band consistent with the slpE gene. K904, K997, K1172 UC1SER and CMS3335 are clinical isolates; Db11, CHASM, CMS376 and Nima are non-clinical isolates strains.
3.2. Induced expression of slpE confers extracellular protease activity upon strain PIC3611 and requires the LipBCD type 1 secretion system
The slpE ORF was cloned under control of an L-arabinose-inducible promoter on vector pMQ125. The resulting plasmid, pMQ516 (pslpE) was moved into S. marcescens strain PIC3611 because it is a very low-protease-producing strain [27] that would easily allow observation of increased protease levels. Induced expression of slpE conferred a ~8-fold increase in extracellular protease activity compared to the vector control (Fig. 3A). This was comparable to expression of genes for serralysin (pprtS) and slpB (pslpB) from the same vector (Fig. 3A). These data support the hypothesis that SlpE from strain K904 is a functional protease.
Fig. 3. Genetic analysis to test whether SlpE requires the LipD T1SS protein for secretion.
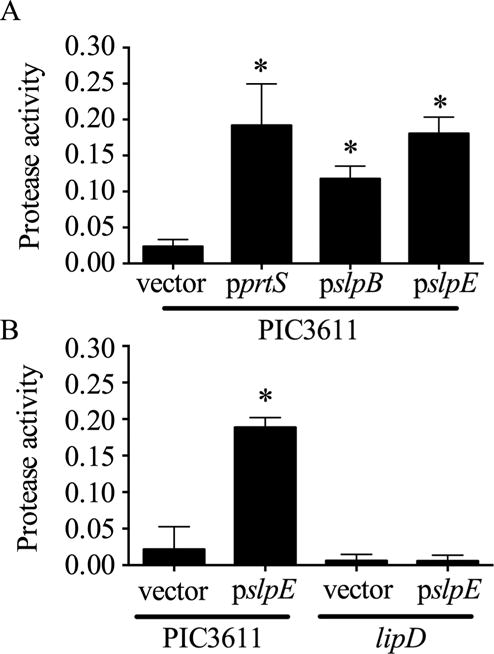
A. Induced expression of slpE or positive controls prtS and slpB from a non-cytotoxic strain conferred cytotoxicity. Asterisk indicates significant difference from the vector control (p<0.01, One Way ANOVA with Tukey’s post-test). B. Secreted protease activity was detected when slpE was induced in the wild type (PIC3611), but not the lipD mutant. Asterisk indicates significant difference from the vector control (p<0.001, One Way ANOVA with Tukey’s post-test). Protease activity is expressed as A440/OD600.
The PrtS and SlpB proteases require the type 1 secretion system composed of LipB, LipC, and LipD (LipBCD) to become extracellular [1], [27]. As a genetic test to determine whether SlpE uses the same secretion system, the slpE gene was induced from pslpE in strain PIC3611 with a lipD mutation. Induced expression of slpE increased extracellular protease activity in growth medium from the wild-type PIC3611 strain, but not in the lipD mutant (Fig. 3B). These genetic data indicate that SlpE export requires the LipBCD type 1 secretion system.
3.3. Expression of slpE is EepR dependent
The transcription factor EepR was recently shown to regulate the prtS and slpB proteases in a positive manner [7]. We tested whether slpE expression was similarly diminished in a ΔeepR strain isogenic to K904 using quantitative RT-PCR. The slpE transcript was measured in cultures grown to OD600=3 (Fig. 4), because Serratia metalloproteases are expressed during stationary phase. There was a significant ~14-fold reduction in the ΔeepR mutant compared to the isogenic wild-type strain (p=0.0076, Student’s t-test, n=6). This result indicates that slpE is regulated by the EepR transcription factor.
Fig. 4. Analysis of slpE transcription in an eepR mutant.
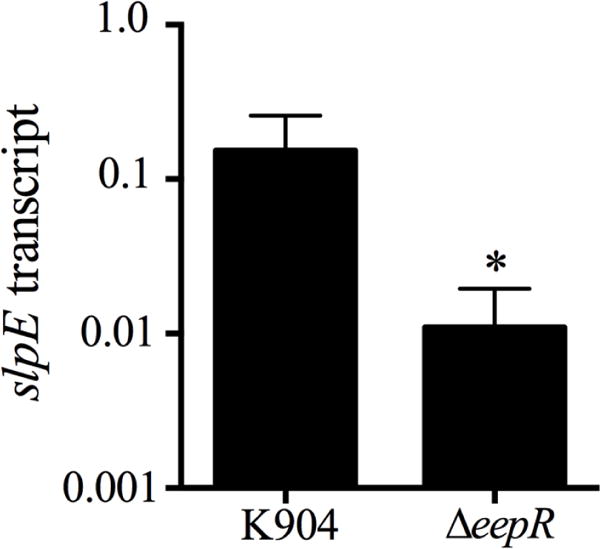
Transcription of slpE was measured in K904 and an isogenic eepR mutant strain at OD600=3. The average and standard deviation of 6 independent samples is shown. The asterisk indicates p=0.0076, Student’s unpaired t-test.
3.4. Calcium-dependent folding of SlpE
To assess the Ca2+-dependent activity of SlpE, the protease was purified and refolded in vitro in the presence or absence of saturating Ca2+ (2 mM). Protease activity was then assessed as a function of digestion of a fluorescently labeled casein substrate. When refolded in the presence of 2 mM Ca2+, the purified SlpE protease showed robust protease activity (Fig. 5). The measured activity of the SlpE protease was similar to, but slightly reduced from, that measured for PrtS and SlpB. These enzymatic activities were dependent on the presence of Ca2+, as proteases refolded in PBS showed minimal hydrolytic activity using the casein assay. This is consistent with prior studies showing that serralysin folding is mediated by Ca2+-binding within the C-terminal RTX domains [27], [33].
Fig. 5. Calcium-dependent structure and activity of SlpE and serralysin proteases.
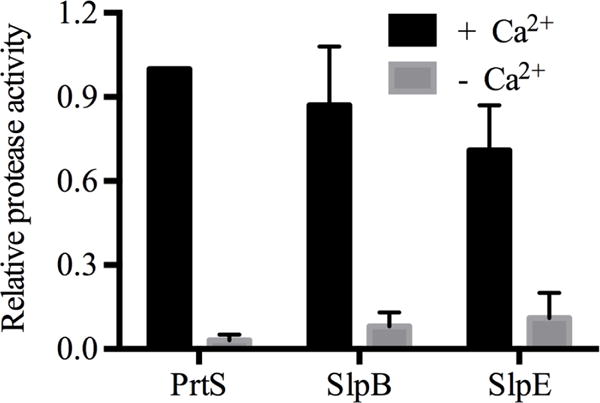
The Ca2+-dependent activation of serralysin and related proteases is shown. Calcium is required for the activity of many proteases; this experiment was performed to determine whether SlpE activity requires calcium. The mean steady state reaction velocities for each protease were normalized to that of PrtS after refolding in the presence or absence of 2 mM Ca2+ using a fluorescent substrate .
3.5. SlpE has redundant function with PrtS and SlpB in killing human airway epithelial cells in vitro
To test whether slpE from strain K904 codes for a cytotoxic protease, pslpE was induced in low-protease-producing strain PIC3611, and normalized bacteria-free culture supernatants were tested for cytotoxicity to a human airway cell line (A549). This cell type is relevant, as S. marcescens is a frequent cause of nosocomial pneumonia [11]. Controls of triton X-100 detergent and PBS were used to establish minimum and maximum values for cell viability (Fig. 6A). Supernatants from strain PIC3611 with the vector control plasmid were not significantly different from the PBS treated cells, demonstrating that supernatants from strain PIC3611 were not cytotoxic under these conditions. Induction of slpE conferred detergent-level cytotoxicity to the PIC3611 supernatants (Fig. 6A), suggesting that SlpE is cytotoxic to human airway cells. Similar results were observed with induction of the positive control prtS (Fig. 6A).
Fig. 6. Genetic evidence supporting slpE codes for a protein cytotoxic to a human carcinoma airway epithelial cell line.
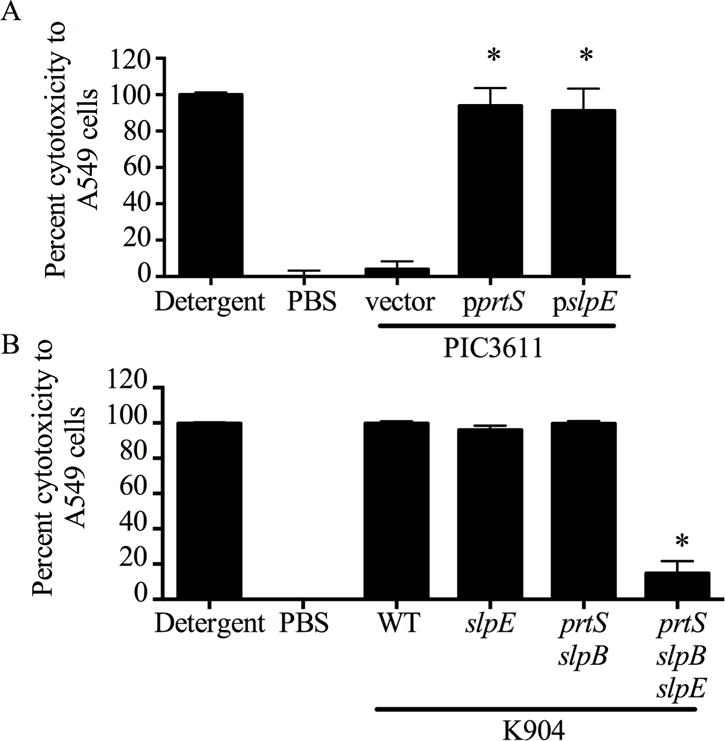
A549 cells were challenged by bacterial culture filtrates for 4 h and viability was measured by Presto Blue. Detergent and PBS act as positive and negative controls, respectively. A. Induced expression of slpE or positive control prtS from a non-cytotoxic strain conferred cytotoxicity. Asterisk indicates significant difference from the vector control (p<0.001, One Way ANOVA with Tukey’s post-test). B. Supernatants from a protease triple mutant (prtS slpB slpE), but not a single or double protease mutants in highly cytotoxic strain K904 are defective in cytotoxicity. WT indicates strain K904. Asterisk indicates significant difference from the WT (p<0.001, One Way ANOVA with Tukey’s post-test).
A high-protease-producing and cytotoxic bacterial strain, K904, was used to test the importance of SlpE in cytotoxicity to A549 airway epithelial cells. After 2 h incubation with normalized bacteria-free supernatants from K904, ~100% cytotoxicity was measured (Fig. 6B), demonstrating that it is much more cytotoxic than strain PIC3611 (Fig. 6A). Mutation of the slpE gene alone did not affect cytotoxicity (Fig. 6B). It was predicted that the cytotoxic impact of an slpE mutation was masked by the PrtS and SlpB proteases. A prtS slpB slpE triple mutant was generated and found to be significantly less cytotoxic to A549 cells than the prtS slpB double mutant (Fig. 6B). Thus, only when redundant protease activity was removed were we able to measure the relative contribution of SlpE to cytotoxicity.
When pslpE was introduced into the prtS slpB slpE mutant and induced, the resulting supernatants had restored protease activity and cytotoxicity (Fig. 7A–B), whereas the vector control did not (Fig. 7A–B). Together, these genetic data indicated that SlpE in combination with PrtS and SlpB contributes to the cytotoxic capacity of the bacterium.
Fig. 7. Complementation of the slpE mutant defects.
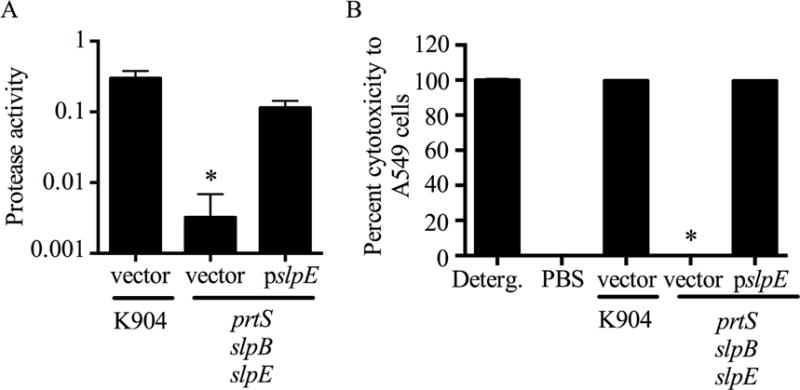
A. Protease activity (A440/OD600) was measured from culture filtrates from strain K904 and a triple protease mutant. The slpE gene on a plasmid restored protease activity. B. A549 cells were challenged by bacterial culture filtrates for 4 h and viability was measured by Presto Blue. Detergent (Deterg.) and PBS act as positive and negative controls, respectively. The slpE gene on a plasmid restored cytotoxicity. Mean and one standard deviation are shown for all bars. Asterisk indicates significantly less protease activity or cytotoxicity compared to the K904 strain (p<0.001, One-way ANOVA with Tukey’s post-test).
4. Discussion
This study has characterized a member of the serralysin family proteases that was strongly associated with clinical isolates rather than non-clinical isolates. In this study, SlpE production did cause cytotoxicity to a human cell line relevant to tissue infected by S. marcescens, suggesting a role for SlpE in virulence. A previous report suggested that SlpB was acquired through a horizontal gene transfer event, as it is more similar in amino acid sequence to AprA from P. aeruginosa than to PrtS [27]. Conversely, the SlpE sequence was found to be highly similar to PrtS (Fig. 1), suggesting that it results from a duplication event. Consistent with this model, structural superposition of the serralysins showed minimal changes in overall architecture (Fig. 1C). The C-terminal RTX domains and the core of the protease domain fold superposed with minimal deviation. Local changes were seen surrounding the active site and may tune the proteases for different substrates or activities. Comparative genomic and phenotypic analyses will be necessary to determine when the slpE gene was introduced into the S. marcescens evolutionary tree, and whether this protease correlates with biotype/genotypes that have acquired a higher capacity for virulence [14].
Mutation of slpE alone did not alter the cytotoxicity of strain K904-secreted fractions; however, a mutation of slpE provided a clear phenotype when mutated from a prtS slpB double mutant, indicating redundant function. The redundancy of SlpE with PrtS and SlpB raises questions regarding whether there is a specific benefit to the bacterium of having multiple similar proteins with redundant function. At present, experiments to compare their specificity, optimal temperature and pH ranges have not been performed and may provide insight into why these multiple genes with apparently similar function are maintained in certain strains of S. marcescens. Additionally, the genes may be differentially regulated and expressed in different ecological niches. Interestingly, a protease-deficient SM6 mutant isolate lost almost all cytotoxicity to HeLa cells in vitro [20]. This suggests that SM6 has one cytotoxic protease compared to K904, which has PrtS, SlpB and SlpE, or that the mutation was in a positive regulator of multiple proteases, as the SM6 protease mutation was never mapped and could have been in a transcriptional regulator. Such regulators may exist, as the EepR gene that positively regulates prtS and slpB expression [7] was postulated to regulate slpE expression. Consistent with this prediction, we observed that slpE expression was highly reduced in an eepR mutant strain. Whereas this data indicates that EepR positively regulates prtS, slpB, and slpE, it is likely that these important secreted proteases are regulated by additional transcription factors.
Previous work by the Benedik group studied a protein with the same sequence from S. marcescens strain SM6 [6]; this current study builds upon the previous study and adds new information. When expressed in Escherichia coli, the SM6 protease was not secreted [6] unless the E. coli hemolysin transporter genes hlyB and hlyD were expressed in trans, indicating that the protease is secreted through a type I secretion system [30]; however, the native S. marcescens type I secretion system used to secrete the SM6 protease was not determined until this current study, which implicates LipBCD as the native secretion system (Fig. 3B). Strain SM6 with an unmapped transposon insertion, presumably in the metalloprotease gene, was less cytotoxic to HeLa cells in vitro, and expression of the SM6 metalloprotease gene from E. coli increased the cytotoxicity of bacterial culture filtrates to HeLa cells [20]. Here we add to the previous studies by demonstrating that the SlpE/SM6 metalloprotease contributes to the pathogenesis of S. marcescens against human airway cells through directed mutation and induced expression of the slpE gene using a modern clinical isolate (Fig. 6B). We demonstrate that SlpE is a calcium-dependent protease, most likely resulting from gene duplication with a prtS progenitor gene, and is found in the majority of clinical isolates tested, suggesting that it contributes to virulence.
In summary, this study has further characterized a serralysin-like protease from the contact-lens-associated keratitis S. marcescens isolate K904. The results from this study suggest that the SlpE protein is a cytotoxic, calcium-dependent member of the serralysin metalloproteases, and is more likely to be found in clinical isolates and may be developed as a diagnostic marker for cytotoxic strains.
Table 2.
Presence or absence of the slpE gene in S. marcescens strains
| Strain | Classification | slpE internal replicona | slpE ORF repliconb | oxyR repliconc |
|---|---|---|---|---|
| CHASM | Environmental | + | ND | + |
| CMS376 | Laboratory | − | − | + |
| CMS3335 | Clinical | + | ND | + |
| Db11 | Insect pathogen | − | − | + |
| HQ | Environmental | − | − | + |
| K904 | Clinical | + | + | + |
| K912 | Clinical | − | − | + |
| K927 | Clinical | + | ND | + |
| K952 | Clinical | + | ND | + |
| K977 | Clinical | + | ND | + |
| K981 | Clinical | + | ND | + |
| K997 | Clinical | + | ND | + |
| K1054 | Clinical | − | − | + |
| K1064 | Clinical | − | − | + |
| K1097 | Clinical | − | + | + |
| K1154 | Clinical | + | ND | + |
| K1172 | Clinical | + | ND | + |
| K1178 | Clinical | + | ND | + |
| K1263 | Clinical | + | ND | + |
| K1265 | Clinical | + | ND | + |
| K1301 | Clinical | + | ND | + |
| K1327 | Clinical | + | ND | + |
| K1467 | Clinical | + | ND | + |
| K1489 | Clinical | + | ND | + |
| K1496 | Clinical | − | − | + |
| K1674 | Clinical | + | ND | + |
| K1681 | Clinical | + | ND | + |
| K1728 | Clinical | + | + | + |
| K1739 | Clinical | − | − | + |
| K1776 | Clinical | + | + | + |
| K1780 | Clinical | + | + | + |
| K1788 | Clinical | − | − | + |
| K1800 | Clinical | − | − | + |
| K1876 | Clinical | − | − | + |
| K1890 | Clinical | + | + | + |
| K2089 | Clinical | + | + | + |
| K2119 | Clinical | − | ND | + |
| K2272 | Clinical | − | − | + |
| K3082 | Clinical | − | ND | + |
| Nima | Laboratory | − | − | + |
| TM | Environmental | − | − | + |
| UCISER | Clinical | + | ND | + |
Primers amplify an internal region of the slpE as an indication of its presence in a genome.
Primers amplify the slpE ORF as an indication of its presence in a genome. These were used with positive controls and if the internal primers failed to amplify slpE. ND = not determined.
The highly conserved oxyR replicon served as a positive control to validate template DNA.
Acknowledgments
The authors thank Kristin Hunt, Benjamin Treat and Kathleen Yates for expert technical assistance. This work was supported by NIH grants AI085570 (to RS), DK083284 (to PT), EY024785 (to KB) and the Eye and the Ear Foundation of Pittsburgh.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare no conflicts of interest.
References
- 1.Akatsuka H, Kawai E, Omori K, Shibatani T. The three genes lipB, lipC, and lipD involved in the extracellular secretion of the Serratia marcescens lipase which lacks an N-terminal signal peptide. J Bacteriol. 1995;177:6381–9. doi: 10.1128/jb.177.22.6381-6389.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bertani G. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J Bacteriol. 1951;62:293–300. doi: 10.1128/jb.62.3.293-300.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biasini M, Bienert S, Waterhouse A, Arnold K, Studer G, Schmidt T, et al. SWISS-MODEL: modelling protein tertiary and quaternary structure using evolutionary information. Nucleic Acids Res. 2014;42:W252–8. doi: 10.1093/nar/gku340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Binet R, Letoffe S, Ghigo JM, Delepelaire P, Wandersman C. Protein secretion by Gram-negative bacterial ABC exporters–a review. Gene. 1997;192:7–11. doi: 10.1016/s0378-1119(96)00829-3. [DOI] [PubMed] [Google Scholar]
- 6.Braunagel SC, Benedik MJ. The metalloprotease gene of Serratia marcescens strain SM6. Mol Gen Genet. 1990;222:446–51. doi: 10.1007/BF00633854. [DOI] [PubMed] [Google Scholar]
- 7.Brothers KM, Stella NA, Romanowski EG, Kowalski RP, Shanks RM. EepR mediates secreted protein production, desiccation survival, and proliferation in a corneal infection model. Infect Immun. 2015 doi: 10.1128/IAI.00466-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Butterworth MB, Zhang L, Liu X, Shanks RM, Thibodeau PH. Modulation of the epithelial sodium channel (ENaC) by bacterial metalloproteases and protease inhibitors. PLoS One. 2014;9:e100313. doi: 10.1371/journal.pone.0100313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cruz-Romero M, Kelly AL, Kerry JP. Influence of packaging strategy on microbiological and biochemical changes in high-pressure-treated oysters. J Sci Food Agric. 2008;88:2713–23. [Google Scholar]
- 10.Delepelaire P. Type I secretion in gram-negative bacteria. Biochim Biophys Acta. 2004;1694:149–61. doi: 10.1016/j.bbamcr.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 11.Gaynes R, Edwards JR. Overview of nosocomial infections caused by gram-negative bacilli. Clin Infect Dis. 2005;41:848–54. doi: 10.1086/432803. [DOI] [PubMed] [Google Scholar]
- 12.Ghigo JM, Wandersman C. A fourth metalloprotease gene in Erwinia chrysanthemi. Res Microbiol. 1992;143:857–67. doi: 10.1016/0923-2508(92)90073-w. [DOI] [PubMed] [Google Scholar]
- 13.Giard DJ, Aaronson SA, Todaro GJ, Arnstein P, Kersey JH, Dosik H, et al. In vitro cultivation of human tumors: establishment of cell lines derived from a series of solid tumors. J Natl Cancer Inst. 1973;51:1417–23. doi: 10.1093/jnci/51.5.1417. [DOI] [PubMed] [Google Scholar]
- 14.Grimont PA, Grimont F. Biotyping of Serratia marcescens and its use in epidemiological studies. J Clin Microbiol. 1978;8:73–83. doi: 10.1128/jcm.8.1.73-83.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hao JH, Sun M. Purification and characterization of a cold alkaline protease from a psychrophilic Pseudomonas aeruginosa HY1215. Appl Biochem Biotechnol. 2015;175:715–22. doi: 10.1007/s12010-014-1315-2. [DOI] [PubMed] [Google Scholar]
- 16.Iguchi A, Nagaya Y, Pradel E, Ooka T, Ogura Y, Katsura K, et al. Genome Evolution and plasticity of Serratia marcescens, an important multidrug-resistant nosocomial pathogen. Genome Biol Evol. 2014;6:2096–110. doi: 10.1093/gbe/evu160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ishii K, Adachi T, Hara T, Hamamoto H, Sekimizu K. Identification of a Serratia marcescens virulence factor that promotes hemolymph bleeding in the silkworm, Bombyx mori. J Invertebr Pathol. 2014;117:61–7. doi: 10.1016/j.jip.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 18.Kalivoda EJ, Stella NA, Aston MA, Fender JE, Thompson PP, Kowalski RP, et al. Cyclic AMP negatively regulates prodigiosin production by Serratia marcescens. Res Microbiol. 2010;161:158–67. doi: 10.1016/j.resmic.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Letoffe S, Ghigo JM, Wandersman C. Identification of two components of the Serratia marcescens metalloprotease transporter: protease SM secretion in Escherichia coli is TolC dependent. J Bacteriol. 1993;175:7321–8. doi: 10.1128/jb.175.22.7321-7328.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marty KB, Williams CL, Guynn LJ, Benedik MJ, Blanke SR. Characterization of a cytotoxic factor in culture filtrates of Serratia marcescens. Infect Immun. 2002;70:1121–8. doi: 10.1128/IAI.70.3.1121-1128.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsumoto K. Role of bacterial proteases in pseudomonal and serratial keratitis. Biol Chem. 2004;385:1007–16. doi: 10.1515/BC.2004.131. [DOI] [PubMed] [Google Scholar]
- 22.Matsumoto K, Maeda H, Takata K, Kamata R, Okamura R. Purification and characterization of four proteases from a clinical isolate of Serratia marcescens kums 3958. J Bacteriol. 1984;157:225–32. doi: 10.1128/jb.157.1.225-232.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramachandran S, Kota P, Ding F, Dokholyan NV. Automated minimization of steric clashes in protein structures. Proteins. 2011;79:261–70. doi: 10.1002/prot.22879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shanks RM, Caiazza NC, Hinsa SM, Toutain CM, O’toole GA. Saccharomyces cerevisiae-based molecular tool kit for manipulation of genes from gram-negative bacteria. Appl Environ Microbiol. 2006;72:5027–36. doi: 10.1128/AEM.00682-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shanks RM, Kadouri DE, Maceachran DP, O’toole GA. New yeast recombineering tools for bacteria. Plasmid. 2009;62:88–97. doi: 10.1016/j.plasmid.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shanks RM, Stella NA, Arena KE, Fender JE. Mutation of crp mediates Serratia marcescens serralysin and global secreted protein production. Res Microbiol. 2013;164:38–45. doi: 10.1016/j.resmic.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shanks RM, Stella NA, Hunt KM, Brothers KM, Zhang L, Thibodeau PH. Identification of SlpB, a cytotoxic protease from Serratia marcescens. Infect Immun. 2015;83:2907–16. doi: 10.1128/IAI.03096-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stella NA, Lahr RM, Brothers KM, Kalivoda EJ, Hunt KM, Kwak DH, et al. Serratia marcescens cyclic AMP-receptor protein controls transcription of EepR, a novel regulator of antimicrobial secondary metabolites. J Bacteriol. 2015;197:2468–2478. doi: 10.1128/JB.00136-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stocker W, Grams F, Baumann U, Reinemer P, Gomis-Ruth FX, Mckay DB, et al. The metzincins-topological and sequential relations between the astacins, adamalysins, serralysins, and matrixins (collagenases) define a superfamily of zinc-peptidases. Protein Sci. 1995;4:823–40. doi: 10.1002/pro.5560040502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suh Y, Benedik MJ. Production of active Serratia marcescens metalloprotease from Escherichia coli by a-hemolysin HlyB and HlyD. J Bacteriol. 1992;174:2361–2366. doi: 10.1128/jb.174.7.2361-2366.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wandersman C, Delepelaire P, Letoffe S, Ghigo JM. A signal peptide-independent protein secretion pathway. Antonie Van Leeuwenhoek. 1992;61:111–3. doi: 10.1007/BF00580616. [DOI] [PubMed] [Google Scholar]
- 32.Zhang L, Conway JF, Thibodeau PH. Calcium-induced folding and stabilization of the Pseudomonas aeruginosa alkaline protease. J Biol Chem. 2012;287:4311–22. doi: 10.1074/jbc.M111.310300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang L, Morrison AJ, Thibodeau PH. Interdomain contacts and the stability of serralysin protease from Serratia marcescens. PLoS ONE. 2015;10:e0138419. doi: 10.1371/journal.pone.0138419. [DOI] [PMC free article] [PubMed] [Google Scholar]


