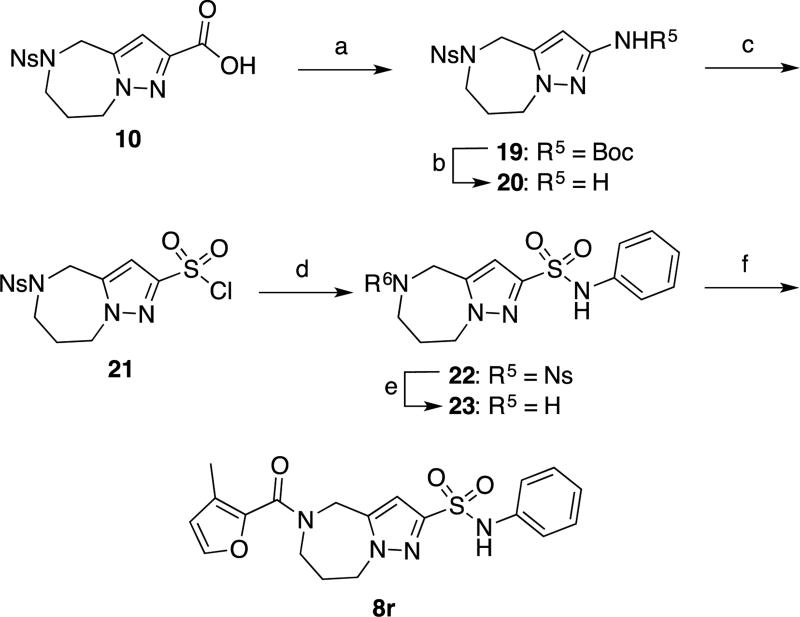
Scheme 4.
Synthesis of compound 8ra
aReagents and conditions (a) DPPA, Et3N, t-BuOH/toluene/1,2-dichloroethane 1.5:1.5:1, RT to reflux, 32%. (b) HCl/dioxane, then NaHCO3, RT, 99%. (c) NaNO2/aq. HCl, then CuSO4, SO2, AcOH/water, −10 °C to RT, 36%. (d) PhNH2, Et3N, THF, RT, 84%. (e) PhSH, Cs2CO3, CH3CN, RT, 79%. (f) EDCI, HOBt, 3-methylfuran-2-carboxylic acid, DMF, RT, 76%.