Figure 1.
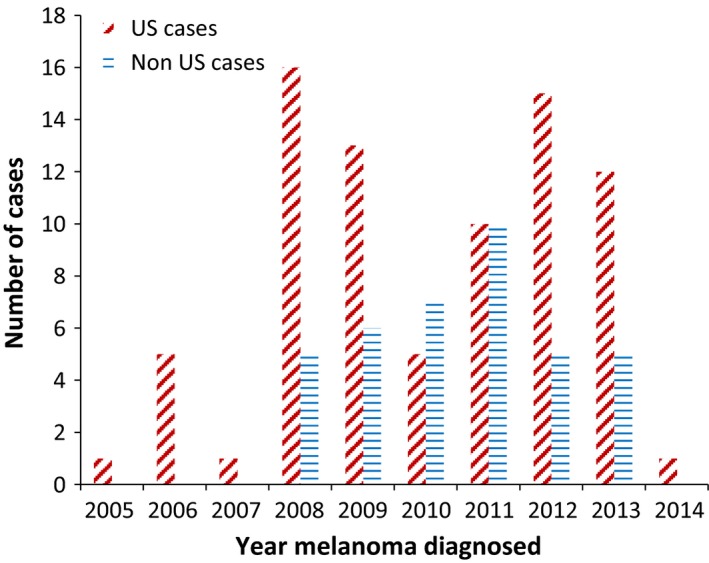
Year when natalizumab‐associated melanoma cases from the United States (US) and other countries (Non US cases) were reported to the Food and Drug Administration. Data source: FDA’s Adverse Event Reporting System (FAERS) database (January 1, 2008‐ March 31, 2014).
