Abstract
AIM
To investigate the long-term effect of dietary education on a low fermentable oligosaccharide, disaccharide and polyol (FODMAP) diet on irritable bowel syndrome (IBS) symptoms and quality of life (QoL).
METHODS
Participants with IBS (Rome III) were randomized to two groups. Group I commenced a low FODMAP diet at baseline. At three months, group II, so far a comparator group, crossed over to a low FODMAP diet while group I started re-challenging foods. All patients completed the IBS SSS (IBS symptom severity scoring system, 0-500 points increasing with severity), IBS QoL questionnaire (0-100 increasing with QoL), a FODMAP specific food frequency questionnaire and provided a stool sample at baseline, three and six months for microbiome analysis.
RESULTS
Fifty participants were enrolled into group I (n = 23) or group II (n = 27). Participants in both groups were similar in baseline values but with more men in group I. There was a significantly lower IBS SSS (275.6 ± 63.6 to 128.8 ± 82.5 vs 246.8 ± 71.1 to 203.6 ± 70.1) (P < 0.0002) and increased QoL (68.5 ± 18.0 to 83 ± 13.4 vs 72.9 ± 12.8 to 73.3 ± 14.4) (P < 0.0001) in group I vs group II at 3 mo. The reduced IBS SSS was sustained at 6 mo in group I (160 ± 102) and replicated in group II (124 ± 76). Fiber intake decreased on the low FODMAP diet (33 ± 17 g/d to 21 ± 8 g/d) (P < 0.01) and after re-introducing FODMAP containing foods increased again to 27 ± 9 g/d. There was no change seen in the intestinal microbiome when participants adopted a low FODMAP diet.
CONCLUSION
This study demonstrated that a reduction in FODMAPs improves symptoms in IBS and this improvement can be maintained while reintroducing FODMAPs.
Keywords: Irritable bowel syndrome, FODMAP, Short chain fermentable carbohydrates, Microbiota, Diet, Microbiome
Core tip: Dietary education by a dietitian on a low FODMAP diet leads to a reduction in symptoms and an improvement in quality of life. Commencing a low FODMAP diet does not appear to alter microbial diversity in patients with irritable bowel syndrome (IBS). Patients with IBS when guided by a dietitian on reintroducing FODMAP containing foods to tolerance are able to increase their intake of fiber to recommended levels without significant worsening of symptoms.
INTRODUCTION
Irritable bowel syndrome (IBS) is a disorder characterized by abdominal discomfort associated with altered bowel function in which no structural or biochemical abnormalities are observed[1]. It is a chronic and remitting condition with symptom severity fluctuating over time[2], a crossover between presentations is also common[3]. Worldwide prevalence was calculated as 11.2% with an Australasian prevalence of 14%[4]. IBS patients have a reduced quality of life; with an increase in severity corresponding with a decreased quality of life[5]. Patients with IBS visit their doctor more frequently and consume more health resources[6]. IBS can be subdivided into diarrhea predominant IBS(D), constipation predominant IBS(C), or mixed IBS(M) with patients experiencing pain[1,7]. The pathogenesis of IBS is multifactorial, heterogeneous and incompletely understood[8]. Dysbiosis, abnormal gut motility, inflammation, an altered brain-gut axis, psychological distress, increased mucosal permeability, impaired immune function and a heightened visceral sensitivity are all thought to play a part in the pathology[9]. Pharmacological management reflects the heterogeneity of IBS with medical management directed at individual symptoms. Therapies commonly utilized include antispasmodics, laxatives, anti-diarrhea medications, opioids, and low dose antidepressants depending on the leading symptom[10]. Cognitive behavioral therapy and hypnotherapy have been shown to be beneficial[11].
Patients with IBS have long identified that eating provokes IBS symptoms and consequently avoid some foods[12]. Interest is increasing within the medical and scientific community regarding the role of food in symptom provocation. A diet low in slowly absorbed or indigestible fermentable short chain carbohydrate or the low fermentable oligosaccharide, disaccharide, monosaccharide and polyol (FODMAP) diet[13] aims to reduce symptom severity by targeting aspects of the pathophysiology of IBS. Due to heightened visceral sensitivity in IBS luminal distension is more likely to cause discomfort and pain[14]. Increases in either gas, liquid or solids in the bowel will cause luminal distension. A reduced load of fermentable carbohydrates in the low FODMAP diet should produce less gas[15]. Furthermore, FODMAP molecules have a small particle size which makes them highly osmotically active drawing water into the colon[16].
Since the original retrospective review of a predecessor of the low FODMAP diet[17] showed a reduction in symptom severity in IBS patients there have been several[15,18-27] studies showing an overall reduction in symptom severity in IBS patients following a low FODMAP diet. In retrospective audits of long term effectiveness (≥ 3 mo), 70%-75% of patients report a sustained symptom reduction.
Furthermore, IBS patients appear to have a decreased intestinal microbial diversity, greater temporal instability and a relative increase in Firmicutes compared to healthy individuals[28,29]. Of the environmental factors, diet has the greatest impact on the microbiome[30]. Four studies[21,27,31,32] have investigated the effect of a low FODMAP diet on the microbiome with no consistent effect demonstrated[21,27,31,32]. No studies have examined microbiome changes after re-challenging FODMAPs to tolerance.
To-date limited data is available on FODMAP restriction[21] when participants were only educated on a low FODMAP diet and its effect on symptom reduction, nutritional adequacy and fiber intake. There is no data available on the effect of FODMAP reintroduction. Our aim was to conduct a randomized controlled trial investigating the long-term effect of dietary education on FODMAP intake, nutritional adequacy, symptom severity and quality of life. Furthermore, we aimed to examine the effect of FODMAP reduction on the gastrointestinal microbiome.
MATERIALS AND METHODS
Subjects
Patients with IBS were recruited through gastroenterology outpatient clinics, GP practices and by advertising. Clinical history was reviewed by the gastroenterologist (MS) who assessed for eligibility according to Rome III criteria[33]. Pre-defined exclusion criteria were coeliac disease, inflammatory bowel disease (IBD), pregnancy or lactation, major abdominal surgery and inability to understand English.
Study protocol
This was a parallel design study with participants randomized to either group I or II. Randomization of numbers was done online (http://www.random.org) by RH. Neither investigators nor participants were blinded to the treatment. Allocation to the treatment was concealed. Group I participants received education immediately, were started on the low FODMAP diet at baseline and started reintroduction of foods at three months. Group II participants were given the intervention (dietary education) in the second three month period. During the initial 3 mo waiting period group II received no dietary education. Data was collected at baseline, 3 mo (main comparison) and 6 mo. The IBS Symptom Severity Score questionnaire (IBS SSS) and IBS Quality of Life questionnaire (IBS QoL) were automated on TeleForm (V10.6, Hewlett Packard, Cardiff, United Kingdom). This study received ethical approval from the Upper South A regional ethics committee URA/11/05/015 and was registered with the Australian New Zealand Clinical Trials Registry #342998.
Dietary analysis
Reduction of FODMAP intake was calculated using the automated version of the FODMAP specific food frequency questionnaire (FFQ)[34]. Data from the FFQ was deemed invalid if participants had a non-physiological energy intake < 2000 kJ/d or ticked the same frequency for every item. Total FODMAP intake was defined as the sum of fructo-oligosaccharides (FOS), galacto-oligosaccharides (GOS), lactose, excess fructose to glucose, sorbitol and mannitol.
Change in symptom severity and QoL
The IBS SSS[35] was used to measure overall change in symptom severity. Scores range from 0-500, with scores < 50 similar to that seen in a non-IBS population. The following definitions were assumed: mild 50-175, moderate 175-300, and severe disease > 300. According to the validation paper, a reduction of ≥ 50 was defined as clinical improvement[35]. The subscales, which included bloating and severity (0-100) and frequency of abdominal pain (days in 10: 0-10) within it were analyzed individually. The IBS QoL[36] was used to measure overall change in quality of life. Scores were standardized so the overall instrument and the subscales all had a range 0-100. A change of 10 was defined as clinically significant[36].
Dietary advice
Dietary advice was provided to individual participants in a standardized fashion by an experienced registered dietitian (RH). At the initial consultation (approx. 1 h duration) all participants were advised to significantly reduce their intake of excess fructose, lactose, sorbitol, mannitol, FOS and GOS. Participants then purchased and prepared their own food. At follow-up consultations of 30 min participants were taught to systematically try to reintroduce FODMAP molecules individually, one follow-up appointment was scheduled and then additional appointments were provided on demand. Written resources were developed based previously published resources by Monash University, Melbourne, Australia[14,37-43].
Fecal assessment and comparison of microbiota with symptom response
Stool samples were collected at baseline, three and six months from participants and within 4 h frozen and stored at -20 °C. DNA was extracted using the MoBio 96-well Soil DNA Extraction kit (MoBio Laboratories Inc., Carlsbad, CA, United States) according to the Earth Microbiome Projects protocols (www.earthmicrobiome.org). Samples were then amplified using primers based on the bacterial/archael primers 515F/806R and amplified, sequenced and analyzed as before[44]. Taxonomy was assigned using the RDP classifier[45] to assign taxonomy to genus level with any taxonomic level with a ≤ 0.80 confidence score assigned “unclassified”. Tests for significance of individual taxa were carried out using ALDEx2 version 0.99[46] and community analysis with the Quantitative Insights into Microbial Ecology package[47].
Statistical analysis
Sample size was calculated to detect a difference of 100 points on the IBS SSS. A difference of 50 points on the IBS SSS (35) is clinically significant, thus 100 points should be highly clinically significant. The mean and SD for moderate IBS in the original validation paper was 243 ± 42 (35). Drop outs were calculated at 20% and an alpha of 0.05 was selected. A power of 80% was selected. Therefore 33 participants were needed in each group. Statistical analysis was performed using Stata v12 (StataCorp LP, Stata Statistical Software, College Station, TX, United States). Data is presented as mean and SD unless otherwise specified. ANCOVA, with baseline as a co-variate, was used to test whether there was an intergroup difference in continuous variables at three months. Linear regression was used to determine if there was a relationship between change in FODMAP intake and change in outcome measures.
RESULTS
Participants were recruited between August 2011 and August 2012.The trial was ended within the constraints of recruiting abilities. From 117 potential participants, 50 participants were enrolled in the study (Figure 1), 23 participants in group I and 27 in group II with significantly more males in group II (P < 0.05) (Table 1). During the intervention, participants had a median of two follow-up appointments (range 1-5).
Figure 1.
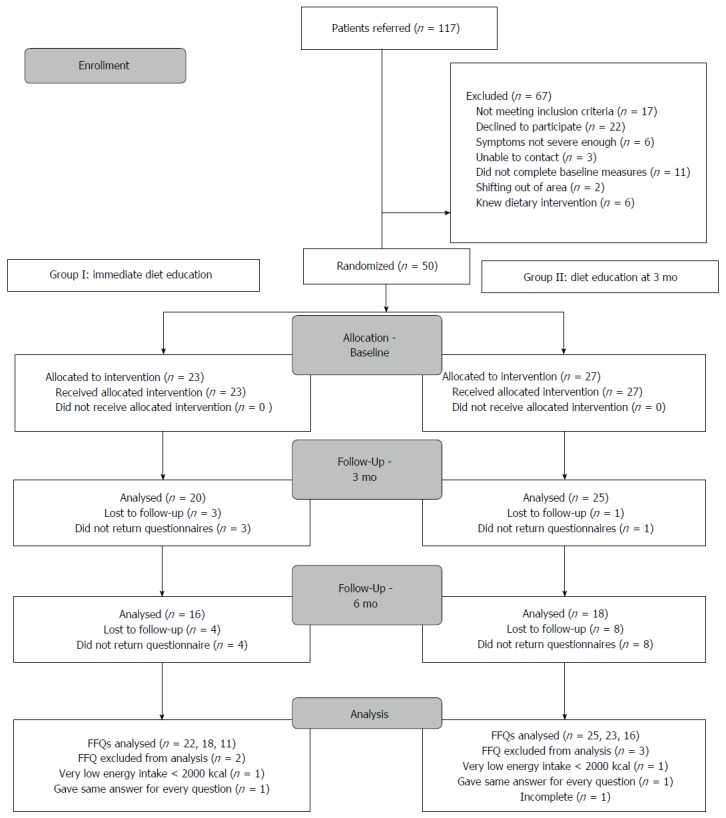
Participant flow.
Table 1.
Baseline demographics n (%)
| Group I (n = 23) | Group II (n = 27) | |
| Age (mean, sd) | 43.3 (13.8) | 40.6 (13.3) |
| Gender | ||
| Male | 6 (26) | 1 (4) |
| Female | 17 (73) | 26 (96) |
| Ethnicity | ||
| Maori | 1 (4) | 1 (4) |
| European | 23 | 26 |
| Type of IBS | ||
| Diarrhoea | 16 (69) | 16 (59) |
| Constipation | 3 (13) | 2 (9) |
| Mixed | 5 (22) | 9 (33) |
| Dietary Pattern | ||
| Minimally restricted diet | 20 | 24 |
| Gluten free | 0 | 2 |
| Lactose free | 2 | 1 |
| Gluten and lactose free | 1 | 0 |
Change in FODMAP intake
At baseline there were no differences in energy intake, macronutrient intake, and total FODMAP intake or between any of the individual FODMAP molecules including lactose between the two groups (Table 2). At 3 mo, there was a significant reduction in reported total energy intake in group I from baseline to 3 mo [2.3 ± 2.9 (s.d) MJ/d, P < 0.01]. There was a 16.5 ± 15.6 g/d (P < 0.01) reduction in total FODMAP intake of participants in group I (Figure 2). There was a significant reduction in all FODMAP molecules individually (Table 2). In group II there were smaller non-significant reductions in total energy intake, FOS and GOS intake during the first three months as expected. The FODMAP intake of group I at 6 mo: 22 ± 11 g was less than at baseline 28 ± 15 g (NS) but greater than at three months 10 ± 10 g (P < 0.01). Thus they had reintroduced the specific FODMAP molecules they tolerated and relaxed restriction on others. In group II there was a significant reduction in total FODMAP intake from 3 mo to 6 mo of 6 ± 8 g (P < 0.02), however, when analyzing the individual FODMAPs only the reduction in lactose was significant between 3 mo and 6 mo 7 ± 10 g (P < 0.01).
Table 2.
FODMAP, fibre and calcium intakes
| Baseline | 3 mo | 6 mo | |
| Total Energy (MJ/d) | |||
| Group I | 10.6 ± 3.5 | 8.4 ± 3.2b | 10.1 ± 2.9d |
| Group II | 10.6 ± 2.8 | 9.7 ± 2.8a | 9.5 ± 2.9 |
| Total FODMAP (g/d) | |||
| Group I | 28 ± 15 | 12 ± 8b | 22 ± 11b |
| Group II | 29 ± 12 | 28 ± 18 | 22 ± 15bc |
| Lactose (g/d) | |||
| Group I | 16 ± 12 | 7 ± 8b | 14 ± 11 |
| Group II | 15 ± 11 | 18 ± 19 | 13 ± 14c |
| Excess Fructose (g/d) | |||
| Group I | 4 ± 3 | 1 ± 1.5a | 2 ± 2a |
| Group II | 6 ± 6 | 4 ± 3 | 3 ± 2a |
| FOS (g/d) | |||
| Group I | 2.6 ± 1.7 | 1.3 ± 0.3b | 1.9 ± 0.8c |
| Group II | 3.0 ± 1.1 | 2.5 ± 0.9a | 2.0 ± 0.8b |
| GOS (g/d) | |||
| Group I | 1.3 ± 0.7 | 0.9 ± 0.7b | 1.3 ± 0.8d |
| Group II | 1.4 ± 1.0 | 1.0 (0.7)b | 1.2 ± 1.1 |
| Sorbitol (g/d) | |||
| Group I | 2.2 ± 1.4 | 0.7 ± 0.4b | 1.1 ± 0.7ac |
| Group II | 3.0 ± 1.3 | 2.9 ± 2.5 | 1.9 ± 1.5a |
| Mannitol (g/d) | |||
| Group I | 1.2 ± 1.3 | 0.4 ± 0.4a | 0.8 ± 0.8 |
| Group II | 0.9 ± 0.3 | 0.8 ± 0.5 | 0.5 ± 0.2b |
| Fiber (g/d) | |||
| Group I | 33 ± 17 | 21 ± 8b | 27 ± 9 |
| Group II | 31 ± 8 | 29 ± 10 | 27 ± 9a |
| Calcium (g/d) | |||
| Group I | 1.1 ± 0.5 | 1.2 ± 0.7 | 1.1 ± 0.5 |
| Group II | 1.0 ± 0.4 | 1.1 ± 0.9 | 1.1 ± 0.6 |
Data is reported as mean ± SD. Total FODMAP is calculated by summing fructo-oligosaccharides (FOS), galacto-oligosaccharides (GOS), lactose, fructose in excess of glucose, sorbitol and mannitol. Data supplied is raw data with no energy adjustment.
P < 0.05,
P < 0.01 vs baseline;
P < 0.05,
P < 0.01 vs 3 mo.
Figure 2.

Comparison of total FODMAP intake between group I who received dietary education immediately after randomisation and began reintroducing FODMAP at three months and group II who received dietary education after the collection of the 3-mo data. Total FODMAP is the sum of galacto-oligosaccharides, fructo-oligosacchardies, lactose, fructose in excess of glucose, sorbitol and mannitol in grams as measured on a FODMAP specific food frequency questionnaire[34]. aP < 0.05, bP < 0.01.
Effect on symptom severity
At baseline there was no difference in IBS SSS in group I (272 ± 60) vs group II (254 ± 80) (P = 0.16). The change in IBS SSS from baseline to 3 mo was statistically significantly larger in group I (-144.5 ± 89.0) than group II (-38.7 ± 74.8) (P < 0.01) (Figure 3). The majority of participants (20) in Group I at 3 mo had scores < 175 indicating mild IBS with three of those having a score < 50 similar to scores seen in a non-IBS population. In group II there were no participants with scores < 50, 10 had mild IBS, 17 had moderate IBS and 3 had severe IBS. Investigating the changes in IBS SSS in each subtype, we found a significant reduction for IBS(D) in group I (114.5 ± 89) (P < 0.01) and group II (89 ± 81) (P < 0.01) during their intervention period and for IBS(M) in group II (112 ± 38) (P = 0.03) during their intervention period. Due to very small numbers sub-analysis was not done for IBS(C) participants. At 6 mo the clinical improvement in IBS was sustained overall in group I despite increasing FODMAP molecules to tolerance (Figure 3). However there were some participants who moved from mild to moderate IBS and one to severe IBS at 6 mo. In group II at 6 mo all participants had scores representative of mild IBS or similar to those of people without IBS.
Figure 3.
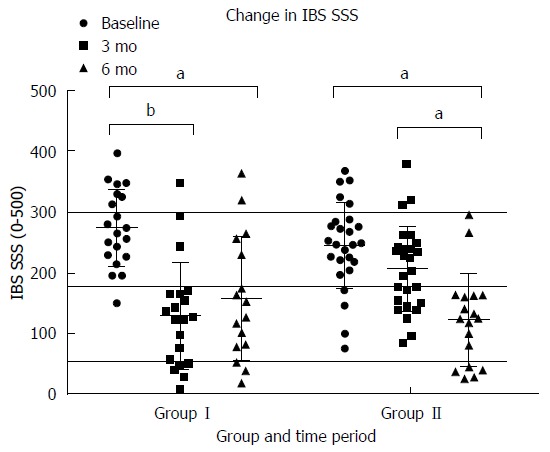
Change in IBS severity scoring system[35] by group and time period. Participants in Group I received dietary education immediately after baseline measures and started reintroductions to tolerance at 3 mo. Participants in group II received dietary education after collection of data at 3 mo. Scores > 300 indicate severe IBS, 175-300 indicate moderate IBS, 50-175 indicate mild IBS and scores < 50 are similar to those of people without IBS. aP < 0.05, bP < 0.01.
At 3 mo there was a statistically significant greater reduction in the maximum number of bowel motions experienced per day in group I (1.7 ± 2.6) vs group II (0.1 ± 1.7) (P < 0.01). This was not seen in group II at six months (Figure 4). At 3 mo there was statistically significantly greater reduction in how often participants experienced pain in group I (3.4 ± 2.9 d in ten) than group II (0.2 ± 1.9 d in ten) (P < 0.01). This was replicated in group II at between three and six months (Figure 5). This reduction in frequency in pain was sustained until six months in group I. No effect of the low FODMAP diet was seen on either severity of pain (Figure 6A) or abdominal distension (Figure 6B) in either group at three months. The reduction in overall symptom severity was replicated with group II in their intervention period (209 ± 80 to 124 ± 76) (P < 0.01). In the intervention period for group II there was a statistically significant reduction in severity of pain (33 ± 26 to 17 ± 17) (P = 0.02), frequency of pain (3.3 ± 2.5 to 1.9 ± 2.1 d in 10) (P = 0.03) and abdominal distension (39 ± 36 to 17 ± 20) (P = 0.01).
Figure 4.
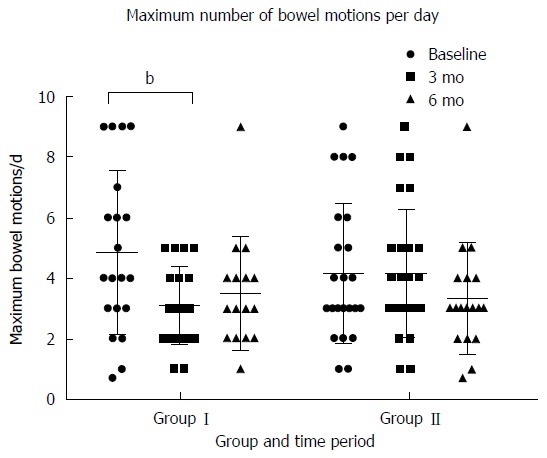
Maximum number of bowel motions reported per day by participants by time period and group. Group I received their dietary education after the collection of baseline measures and started reintroducing FODMAP to tolerance at 3 mo. Group II received their dietary education after the collection of data at 3 mo. bP < 0.01.
Figure 5.
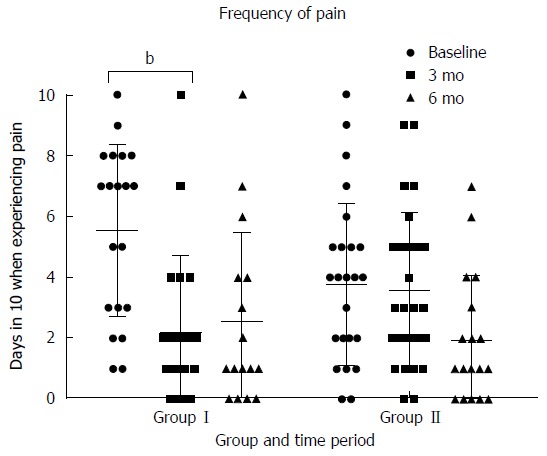
Days in ten when participants were experiencing pain by time period and group. Participants in Group I received dietary education after collection of baseline measures and at 3 mo were encouraged to reintroduce FODMAP foods to tolerance. Participants in Group II received dietary education after the collection of data at 3 mo. bP < 0.01.
Figure 6.
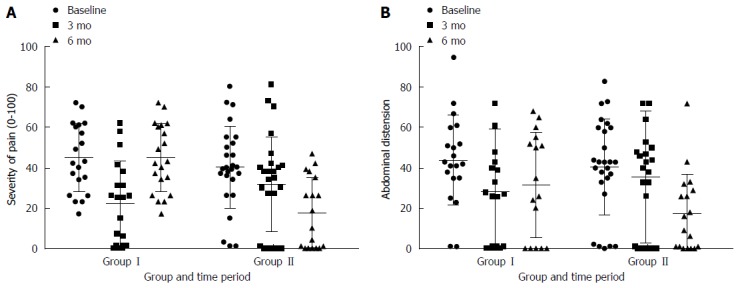
Change in severity of pain (A) and abdominal distension (B) by group and time period. Increasing scores represent increasing severity. This is a subscale of the irritable bowel syndrome symptom severity scoring system[34]. Participants in group I received dietary education immediately after collecting baseline measures and after 3 mo were reintroducing FODMAPs to tolerance. Participants in Group II received dietary education after the data collection at 3 mo.
Effect on QoL
At baseline there was no difference between groups in either the overall IBS QoL (P = 0.26) (Figure 7). At 3 mo there was a clinically (≥ 10 units) and statistically significant greater improvement in IBS related quality of life in group I (66 ± 15 to 81 ± 14) vs group II (73 ± 11 to 73 ± 13) (P < 0.05) (Figure 7). This improvement in IBS QoL of life was sustained at 6 mo in Group I (81 ± 14 to 77 ± 17) (P = 0.1) and replicated in group II (73 ± 13 to 80 ± 12) ( P < 0.01). The only subscale that did not improve in Group I from baseline to 3 mo was food avoidance and the only improvement that was not sustained in Group I was impact on sexual relationships. In Group II the only subscales that improved from baseline to 3 mo were social reaction and relationships. In Group II during their intervention period health worry and food avoidance did not improve.
Figure 7.
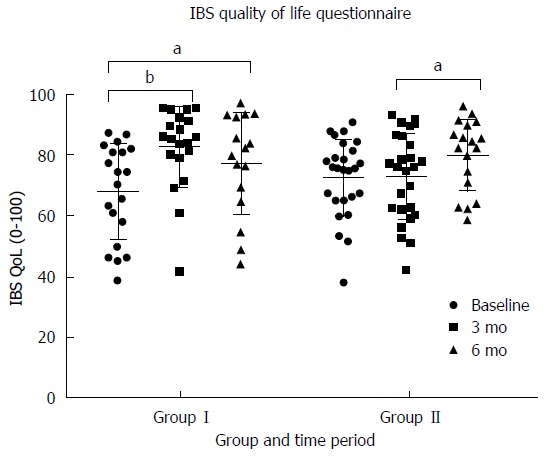
Change in irritable bowel syndrome related quality of life[36] by time period and group. Participants in group I received dietary education after the collection of baseline measures and started reintroducing FODMAP to tolerance after collecting of data at 3 mo. Group II received their dietary education after the collection of data at 3 mo. aP < 0.05, bP < 0.01.
Effect on nutritional adequacy
At baseline there was no difference between the two groups in energy, protein, fat, carbohydrate, and fiber or calcium intake. There was an apparent reduction in energy intake in both Group I (10.6 ± 3.5 MJ to 8.4 ± 3.2 MJ) (P < 0.01) and Group II (10.6 ± 2.8 MJ to 9.7 ± 2.8) (P = 0.03) between baseline and 3 mo with the reduction greater in Group I whose energy intake then increased after reintroduction of FODMAP molecules to tolerance at 6 mo (10.1 ± 2.9 MJ) (P < 0.01). Calcium intake was comparable in both group I (1.1 ± 0.5 g to 1.1 ± 0.7 g) and group II (1.0 ± 0.4 g to 1.0 ± 0.7 g) (P = 0.89) at 3 mo and this was sustained at 6 mo in both group I (1.1 ± 0.5 g) and group II (1.1 ± 0.6 g) (Table 2). All of these intakes exceeded the NZ estimated average requirements (EAR)[48]. There was a reduction in fiber intake in Group I between baseline and 3 mo (32.6 ± 13.9 g to 20.7 ± 11.1g) (Figure 8). This is below the NZ EAR of 30g for adult males and 25 g for adult females[48]. After re-introducing FODMAP to tolerance fiber intakes increased to levels similar to pre-intervention. In Group II the reduction in fiber intake 29 ± 10 g to 27 ± 9 g from 3 mo to 6 mo was not significant.
Figure 8.
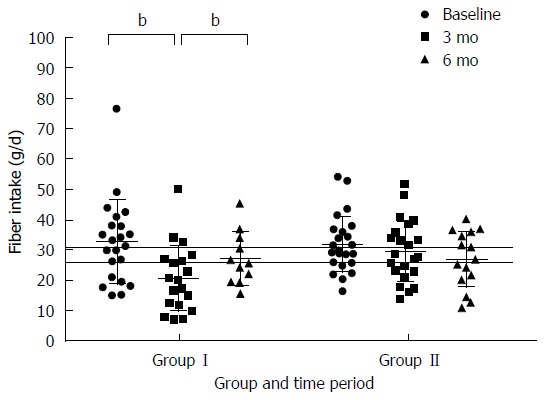
Comparison of total fiber intake between group I who received dietary education immediately after randomisation and began reintroducing FODMAP at three months and group II who received dietary education after the collection of the 3-mo data. Fiber intake was measured on a food frequency questionnaire[34] previously validated for estimating fibre intakes. Recommended fiber intakes for NZ adult males are 30 g per day and for adult NZ females are 25 g per day as represented by the horizontal lines, bP < 0.01.
Change in microbiota
Whole stool samples were obtained from participants in sterile containers, within 4 h of collection they were frozen at -20 °C. Sequences of the V4 region of 16S rRNA gene were obtained from 107 fecal samples. Of these, two were discarded due to low sequencing coverage (< 9000 reads). This yielded 105 samples with a mean number of reads per sample of 27518 ± 6887 SD (range: 14143-45584) providing high sequencing depth per sample. At a 97% clustering identity and a minimum 1% abundance in at least one sample, 244 operational taxonomic units (OTUs) were observed.
Due to an electrical failure a subset (36/107) of early samples were accidentally thawed. This corresponded to 32/48 samples from the baseline visit and 4/37 from the 3 mo visit. The effect of thawing resulted in an obvious skew to the microbiota profiles (Supplementary Figure 1) which was statistically significant (P = 0.001, R = 0.44 ANOSIM) rendering these samples uninformative for analysis.
None the less, no obvious differences were observed in unaffected samples after dietary intervention in Group II (Supplementary Figure 2) and there were no changes in alpha diversity (Figure 9). Using a paired analysis of Group II participants before and after intervention (n = 12 per timepoint), no OTUs were found to have been significantly altered by dietary intervention (FDR-corrected paired Welch’s t-test < 0.05).
Figure 9.
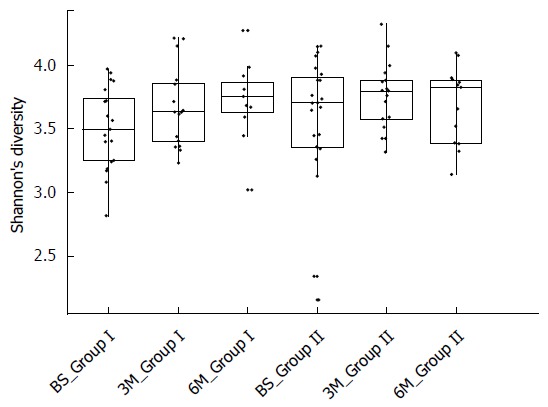
Diversity of samples measured by the Shannon index. Participants in Group I commenced the low FODMAP diet after collection of the baseline measures and in Group II after the collection of data at three months. Each sample is represented by one dot.
Given the heterogeneity of human responses we carried out an exploratory subgroup analysis to determine microbes present after intervention, which may be predictive of positive or negative outcomes from the FODMAP diet. A responder was defined as an individual showing an improvement in IBS severity score of at least 200 points while a non-responder showed either no improvement or an improvement of less than 50 points which was based on the classifications of Francis et al[35] where this was the threshold for reliably indicating improvement in disease status. This yielded 6 non-responders (mean improvement = 15.6 ± 25.2 SD) and 4 responders (mean improvement = 258.0 ± 17.8 SD). No differences were observed in alpha, beta diversity and no significant OTUs were identified (data not shown).
DISCUSSION
This real-world and long-term study adds to the growing body of evidence that a dietitian delivered low FODMAP education is effective[17,19-23] for reducing symptom severity in IBS patients. We demonstrated that an overall reduction in the amount of FODMAPs consumed, symptoms and quality of life significantly improved and this was sustained over the six month study period. Furthermore, our results are consistent with previous findings in that the low FODMAP diet is most effective for IBS(D) patients[25]. However, we were unable to demonstrate an effect of the low FODMAP diet on the composition of the intestinal microbiome, although we did see that there was no change in overall diversity when commencing the low FODMAP diet.
One of the concerns raised about a low FODMAP diet is that by reducing GOS and FOS fiber intakes are reduced. As this study collected dietary data before dietary intervention, after the initial intense phase and after structured food re-challenges we were able to demonstrate a reduction in the energy consumption and especially the fiber intake to below recommended amounts during the intense phase of the study. However, with the reintroduction of FODMAP, especially the galacto-oligosaccharides and fructo-oligosaccharides, foods to tolerance the fiber intake increased and the food consumption became nutritionally adequate again. Intake of galacto-oligosaccharides which include legumes, high FODMAP nuts and some vegetables returned to pre-dietary intervention levels. Fiber is an important substrate for bacteria and their fermentation not only inhibits the growth of pathobionts but also produces short chain fatty acids (SCFA)[49] and is associated with microbial diversity. SCFA are an energy source for the colonocytes and play a regulatory role affecting trans epithelial fluid transport[50], decreased inflammation[51], oxidative stress[52], increases epithelial tight junctions[53] and increases intestinal motility[54] and are therefore central to presumed pathomechanisms leading to IBS. After re-challenging, GOS intakes increased to pre dietary intervention levels. As GOS have been shown in an in vitro colonic model to reduce the production of putrefactive metabolites[55] showing that these FODMAPs were successfully reintroduced during re-challenging is important. This important finding highlights the need for this diet to be supervised by an experienced dietitian, especially during the re-challenge phase.
Similar to Chumpitazi et al[56] and McIntosh et al[27] and Halmos et al[24] we found that a low FODMAP diet did not reduce diversity of the microbiome. Like McIntosh et al[27] participants in our study predominantly had diarrhea. Possibly the effect of increasing microbial diversity with increased transit time[57] compensated for the effect of reduced fiber substrates. With a smaller sample size and a conservative analytic approach our study did not replicate the results by McIntosh et al[27] which saw some changes in the microbiome when IBS patients commenced a low FODMAP diet. Due to the natural inter-personal wide variations in the composition of the microbiome which can have a larger difference than the effect of the dietary intervention[58] it is important that larger studies where samples are collected, stored and processed and analyzed in a consistent manner[59] using appropriate computational biology tools[60]. Furthermore, 16S rRNA sequencing of the gastrointestinal microbiome is only able to detect differences down to the level of operational taxonomic units, whereas functional differences vary by species or even strains. Further studies could aim to use shotgun metagenomic sequencing to study differences in functional capacity of the gut microbiota[61]. Increased levels of some Ruminococcae have been found in greater abundance levels in IBS patients vs healthy controls. Species level increases in the relative abundances of members of the Ruminococcus family have been found for Ruminococcus torques et rel[62-65], another found an increase in Ruminococcus bromii[66], another two found an increase in Ruminococcus gnavus et rel[66,67] and another an increase in the relative abundance of Ruminococcus lactaris[67]. Ideally, future studies of the microbiome should be supported by targeted qPCR of bacteria known to differ between IBS patients and healthy controls. The wide natural variation in the microbiome combined with an infrastructure failure meant we were unable to detect a change in the composition and diversity of the microbiome. Future dietary interventions investigating the effect of diet on the microbiome may benefit from including metabolomics[68]. McIntosh et al[27] found greater separation between a low FODMAP and a high FODMAP diet in the metabolome than the microbiome.
Our study has shown that dietitian delivered dietary education during the re-challenge phase of the diet leads to improved fiber intake without significant worsening of symptoms. Similar to other studies we demonstrated an overall reduction in symptom severity[17,19-22,25,26], a reduction in bloating[19,21,22] and frequency of pain. Two of the three other studies which investigated the effect of a low FODMAP diet on quality of life also showed an improvement[20,25]. Other studies reported a reduction in flatulence[19,21,22], nausea[19,22] and improvement in energy levels[19,21] however, these symptoms were not included in the IBS SSS we used.
A strength of our study was the use of a comparator group as it allowed us to control for the natural fluctuations over time in symptoms severity in patients with IBS. Consequently, the placebo response is high in studies of IBS patients[69]. As seen in this study, there was some improvement in individual participants in group II prior to intervention. While a waiting list comparator group is able to account for the fluctuating nature of symptoms, it is not a true placebo arm as participants are aware of their group allocation and are not expecting to get better and participants received less attention from the study investigators than those in group I. A previous study had shown that in IBS patients the patient-practitioner relationship had evoked the treatment response[70] Data was collected by the dietitian who delivered the dietary education so results could also have been skewed by a desire in participants to “help” the investigator[71].
In conclusion, our study showed that a dietitian delivered low FODMAP education was able to reduce symptom severity and improve quality of life in a group of IBS patients, which was sustained over a six months period. Our study also showed that while fiber intakes decrease initially, after re-challenging they return to a level similar to that prior to dietary intervention further highlighting the need for this dietary intervention to be dietitian-led to monitor and counteract potential nutritional inadequacies.
Further research needs to be conducted to examine the effects of the low FODMAP diet on microbiome and metabolomic data during the intensive phase of the low FODMAP diet but also after patients have re-introduced foods to tolerance. This will provide evidence of the long-term effect of the diet. Including both microbiome and metabolomics will provide information on how the structure, diversity and function of the gastrointestinal microbiome is altered with this dietary change. This will help us to answer the unanswered questions on the long term effects of this diet.
COMMENTS
Background
A low FODMAP diet has been shown to be effective in reducing symptoms in approximately 70% of patients with irritable bowel syndrome (IBS). It is important to understand the potential long term impact of this diet.
Research frontiers
Gastroenterologists and dietitians are interested to learn how a low FODMAP diet may affect the gastrointestinal microbiome because of the role of the microbiome in human health.
Innovations and breakthroughs
This study evaluated the microbiome of IBS patients when reducing FODMAP foods for symptom improvement and after they had been reintroduced to tolerance.
Applications
This study showed that reintroducing FODMAP molecules to tolerance provides the opportunity for IBS patients to meet their fiber intakes.
Terminology
Microbiome: the ecological community of micro-organisms that share our body space; Metabolome: the small molecules produced by the microbiome.
Peer-review
This is a really interesting paper dealing with the possible long-term effect of a low FODMAP diet on IBS patients and stressing the paramount importance of a skilled nutritionist in not only reaching positive results during this kind of diet but also in carefully reintroducing many (not all) FODMAP foods.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: New Zealand
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: This study was reviewed and approved by the Upper South Regional Ethics Committee, New Zealand (#URA/11/05/2011).
Clinical trial registration statement: This study is registered at http://www.anzctr.org.au/. The registration identification number is ANZCTR342998.
Informed consent statement: All study participants provided informed written consent prior to study enrollment.
Conflict-of-interest statement: All authors report no conflicts of interest.
Data sharing statement: Demultiplexed sequence and associated metadata was deposited in the NCBI short read archive with BioProject accession PRJNA392762.
Peer-review started: November 18, 2016
First decision: December 19, 2016
Article in press: March 2, 2017
P- Reviewer: Bellini M, Gibson PR, Ierardi E, Soares RL S- Editor: Gong ZM L- Editor: A E- Editor: Wang CH
References
- 1.American College of Gastroenterology Task Force on Irritable Bowel Syndrome, Brandt LJ, Chey WD, Foxx-Orenstein AE, Schiller LR, Schoenfeld PS, Spiegel BM, Talley NJ, Quigley EM. An evidence-based position statement on the management of irritable bowel syndrome. Am J Gastroenterol. 2009;104 Suppl 1:S1–S35. doi: 10.1038/ajg.2008.122. [DOI] [PubMed] [Google Scholar]
- 2.Chey WD, Kurlander J, Eswaran S. Irritable bowel syndrome: a clinical review. JAMA. 2015;313:949–958. doi: 10.1001/jama.2015.0954. [DOI] [PubMed] [Google Scholar]
- 3.Palsson OS, Baggish JS, Turner MJ, Whitehead WE. IBS patients show frequent fluctuations between loose/watery and hard/lumpy stools: implications for treatment. Am J Gastroenterol. 2012;107:286–295. doi: 10.1038/ajg.2011.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol. 2012;10:712–721.e4. doi: 10.1016/j.cgh.2012.02.029. [DOI] [PubMed] [Google Scholar]
- 5.Gralnek IM, Hays RD, Kilbourne A, Naliboff B, Mayer EA. The impact of irritable bowel syndrome on health-related quality of life. Gastroenterology. 2000;119:654–660. doi: 10.1053/gast.2000.16484. [DOI] [PubMed] [Google Scholar]
- 6.Nyrop KA, Palsson OS, Levy RL, Von Korff M, Feld AD, Turner MJ, Whitehead WE. Costs of health care for irritable bowel syndrome, chronic constipation, functional diarrhoea and functional abdominal pain. Aliment Pharmacol Ther. 2007;26:237–248. doi: 10.1111/j.1365-2036.2007.03370.x. [DOI] [PubMed] [Google Scholar]
- 7.Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130:1480–1491. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 8.Bellini M, Gambaccini D, Stasi C, Urbano MT, Marchi S, Usai-Satta P. Irritable bowel syndrome: a disease still searching for pathogenesis, diagnosis and therapy. World J Gastroenterol. 2014;20:8807–8820. doi: 10.3748/wjg.v20.i27.8807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Camilleri M. Peripheral mechanisms in irritable bowel syndrome. N Engl J Med. 2012;367:1626–1635. doi: 10.1056/NEJMra1207068. [DOI] [PubMed] [Google Scholar]
- 10.Weinberg DS, Smalley W, Heidelbaugh JJ, Sultan S; Amercian Gastroenterological Association. American Gastroenterological Association Institute Guideline on the pharmacological management of irritable bowel syndrome. Gastroenterology. 2014;147:1146–1148. doi: 10.1053/j.gastro.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 11.Ford AC, Quigley EM, Lacy BE, Lembo AJ, Saito YA, Schiller LR, Soffer EE, Spiegel BM, Moayyedi P. Effect of antidepressants and psychological therapies, including hypnotherapy, in irritable bowel syndrome: systematic review and meta-analysis. Am J Gastroenterol. 2014;109:1350–1365; quiz 1366. doi: 10.1038/ajg.2014.148. [DOI] [PubMed] [Google Scholar]
- 12.Halpert A, Dalton CB, Palsson O, Morris C, Hu Y, Bangdiwala S, Hankins J, Norton N, Drossman D. What patients know about irritable bowel syndrome (IBS) and what they would like to know. National Survey on Patient Educational Needs in IBS and development and validation of the Patient Educational Needs Questionnaire (PEQ) Am J Gastroenterol. 2007;102:1972–1982. doi: 10.1111/j.1572-0241.2007.01254.x. [DOI] [PubMed] [Google Scholar]
- 13.Gibson PR, Shepherd SJ. Personal view: food for thought--western lifestyle and susceptibility to Crohn’s disease. The FODMAP hypothesis. Aliment Pharmacol Ther. 2005;21:1399–1409. doi: 10.1111/j.1365-2036.2005.02506.x. [DOI] [PubMed] [Google Scholar]
- 14.Gibson PR, Shepherd SJ. Evidence-based dietary management of functional gastrointestinal symptoms: The FODMAP approach. J Gastroenterol Hepatol. 2010;25:252–258. doi: 10.1111/j.1440-1746.2009.06149.x. [DOI] [PubMed] [Google Scholar]
- 15.Ong DK, Mitchell SB, Barrett JS, Shepherd SJ, Irving PM, Biesiekierski JR, Smith S, Gibson PR, Muir JG. Manipulation of dietary short chain carbohydrates alters the pattern of gas production and genesis of symptoms in irritable bowel syndrome. J Gastroenterol Hepatol. 2010;25:1366–1373. doi: 10.1111/j.1440-1746.2010.06370.x. [DOI] [PubMed] [Google Scholar]
- 16.Barrett JS, Gearry RB, Muir JG, Irving PM, Rose R, Rosella O, Haines ML, Shepherd SJ, Gibson PR. Dietary poorly absorbed, short-chain carbohydrates increase delivery of water and fermentable substrates to the proximal colon. Aliment Pharmacol Ther. 2010;31:874–882. doi: 10.1111/j.1365-2036.2010.04237.x. [DOI] [PubMed] [Google Scholar]
- 17.Shepherd SJ, Gibson PR. Fructose malabsorption and symptoms of irritable bowel syndrome: guidelines for effective dietary management. J Am Diet Assoc. 2006;106:1631–1639. doi: 10.1016/j.jada.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 18.Shepherd SJ, Parker FC, Muir JG, Gibson PR. Dietary triggers of abdominal symptoms in patients with irritable bowel syndrome: randomized placebo-controlled evidence. Clin Gastroenterol Hepatol. 2008;6:765–771. doi: 10.1016/j.cgh.2008.02.058. [DOI] [PubMed] [Google Scholar]
- 19.Staudacher HM, Whelan K, Irving PM, Lomer MC. Comparison of symptom response following advice for a diet low in fermentable carbohydrates (FODMAPs) versus standard dietary advice in patients with irritable bowel syndrome. J Hum Nutr Diet. 2011;24:487–495. doi: 10.1111/j.1365-277X.2011.01162.x. [DOI] [PubMed] [Google Scholar]
- 20.Ostgaard H, Hausken T, Gundersen D, El-Salhy M. Diet and effects of diet management on quality of life and symptoms in patients with irritable bowel syndrome. Mol Med Rep. 2012;5:1382–1390. doi: 10.3892/mmr.2012.843. [DOI] [PubMed] [Google Scholar]
- 21.Staudacher HM, Lomer MC, Anderson JL, Barrett JS, Muir JG, Irving PM, Whelan K. Fermentable carbohydrate restriction reduces luminal bifidobacteria and gastrointestinal symptoms in patients with irritable bowel syndrome. J Nutr. 2012;142:1510–1518. doi: 10.3945/jn.112.159285. [DOI] [PubMed] [Google Scholar]
- 22.de Roest RH, Dobbs BR, Chapman BA, Batman B, O’Brien LA, Leeper JA, Hebblethwaite CR, Gearry RB. The low FODMAP diet improves gastrointestinal symptoms in patients with irritable bowel syndrome: a prospective study. Int J Clin Pract. 2013;67:895–903. doi: 10.1111/ijcp.12128. [DOI] [PubMed] [Google Scholar]
- 23.Wilder-Smith CH, Materna A, Wermelinger C, Schuler J. Fructose and lactose intolerance and malabsorption testing: the relationship with symptoms in functional gastrointestinal disorders. Aliment Pharmacol Ther. 2013;37:1074–1083. doi: 10.1111/apt.12306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Halmos EP, Power VA, Shepherd SJ, Gibson PR, Muir JG. A diet low in FODMAPs reduces symptoms of irritable bowel syndrome. Gastroenterology. 2014;146:67–75.e5. doi: 10.1053/j.gastro.2013.09.046. [DOI] [PubMed] [Google Scholar]
- 25.Pedersen N, Andersen NN, Végh Z, Jensen L, Ankersen DV, Felding M, Simonsen MH, Burisch J, Munkholm P. Ehealth: low FODMAP diet vs Lactobacillus rhamnosus GG in irritable bowel syndrome. World J Gastroenterol. 2014;20:16215–16226. doi: 10.3748/wjg.v20.i43.16215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pedersen N, Vegh Z, Burisch J, Jensen L, Ankersen DV, Felding M, Andersen NN, Munkholm P. Ehealth monitoring in irritable bowel syndrome patients treated with low fermentable oligo-, di-, mono-saccharides and polyols diet. World J Gastroenterol. 2014;20:6680–6684. doi: 10.3748/wjg.v20.i21.6680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McIntosh K, Reed DE, Schneider T, Dang F, Keshteli AH, De Palma G, Madsen K, Bercik P, Vanner S. FODMAPs alter symptoms and the metabolome of patients with IBS: a randomised controlled trial. Gut. 2017;66:1241–1251. doi: 10.1136/gutjnl-2015-311339. [DOI] [PubMed] [Google Scholar]
- 28.Dupont HL. Review article: evidence for the role of gut microbiota in irritable bowel syndrome and its potential influence on therapeutic targets. Aliment Pharmacol Ther. 2014;39:1033–1042. doi: 10.1111/apt.12728. [DOI] [PubMed] [Google Scholar]
- 29.Collins SM. A role for the gut microbiota in IBS. Nat Rev Gastroenterol Hepatol. 2014;11:497–505. doi: 10.1038/nrgastro.2014.40. [DOI] [PubMed] [Google Scholar]
- 30.Xu Z, Knight R. Dietary effects on human gut microbiome diversity. Br J Nutr. 2015;113 Suppl:S1–S5. doi: 10.1017/S0007114514004127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chumpitazi BP, Cope JL, Hollister EB, Tsai CM, McMeans AR, Luna RA, Versalovic J, Shulman RJ. Randomised clinical trial: gut microbiome biomarkers are associated with clinical response to a low FODMAP diet in children with the irritable bowel syndrome. Aliment Pharmacol Ther. 2015;42:418–427. doi: 10.1111/apt.13286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Halmos EP, Christophersen CT, Bird AR, Shepherd SJ, Gibson PR, Muir JG. Diets that differ in their FODMAP content alter the colonic luminal microenvironment. Gut. 2015;64:93–100. doi: 10.1136/gutjnl-2014-307264. [DOI] [PubMed] [Google Scholar]
- 33.Design of Treatment Trials Committee, Irvine EJ, Whitehead WE, Chey WD, Matsueda K, Shaw M, Talley NJ, Veldhuyzen van Zanten SJ. Design of treatment trials for functional gastrointestinal disorders. Gastroenterology. 2006;130:1538–1551. doi: 10.1053/j.gastro.2005.11.058. [DOI] [PubMed] [Google Scholar]
- 34.Barrett JS, Gibson PR. Development and validation of a comprehensive semi-quantitative food frequency questionnaire that includes FODMAP intake and glycemic index. J Am Diet Assoc. 2010;110:1469–1476. doi: 10.1016/j.jada.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 35.Francis CY, Morris J, Whorwell PJ. The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther. 1997;11:395–402. doi: 10.1046/j.1365-2036.1997.142318000.x. [DOI] [PubMed] [Google Scholar]
- 36.Patrick DL, Drossman DA, Frederick IO, DiCesare J, Puder KL. Quality of life in persons with irritable bowel syndrome: development and validation of a new measure. Dig Dis Sci. 1998;43:400–411. doi: 10.1023/a:1018831127942. [DOI] [PubMed] [Google Scholar]
- 37.Muir JG, Rose R, Rosella O, Liels K, Barrett JS, Shepherd SJ, Gibson PR. Measurement of short-chain carbohydrates in common Australian vegetables and fruits by high-performance liquid chromatography (HPLC) J Agric Food Chem. 2009;57:554–565. doi: 10.1021/jf802700e. [DOI] [PubMed] [Google Scholar]
- 38.Shepherd SJ, Gibson P. Food intolerance management plan. Australia: Penguin Group; 2011. [Google Scholar]
- 39.Muir JG, Shepherd SJ, Rosella O, Rose R, Barrett JS, Gibson PR. Fructan and free fructose content of common Australian vegetables and fruit. J Agric Food Chem. 2007;55:6619–6627. doi: 10.1021/jf070623x. [DOI] [PubMed] [Google Scholar]
- 40.Biesiekierski JR, Rosella O, Rose R, Liels K, Barrett JS, Shepherd SJ, Gibson PR, Muir JG. Quantification of fructans, galacto-oligosacharides and other short-chain carbohydrates in processed grains and cereals. J Hum Nutr Diet. 2011;24:154–176. doi: 10.1111/j.1365-277X.2010.01139.x. [DOI] [PubMed] [Google Scholar]
- 41.Barrett J, Glbson P. Clinical ramifications of malabsorption of fructose and other short-chain carbohydrates. Pract Gastroenterol. 2007;31:51–65. [Google Scholar]
- 42.Shepherd S. Low FODMAP diet fructose malabsorption food shopping guide. 5th ed. Australia: Shepherd Works Pty Ltd 2010, 2010 [Google Scholar]
- 43.Gibson PR, Muir J, Barrett JS, Shepherd S, Rose R, Rosella N, Liels K, Yao CK, Biesiekierski J, Halmos EP, et al. The low FODMAP diet. In: Eastern Health Clinical School - Monash University, editor. 1st ed. Melbourne, 2011 [Google Scholar]
- 44.Bisanz JE, Seney S, McMillan A, Vongsa R, Koenig D, Wong L, Dvoracek B, Gloor GB, Sumarah M, Ford B, et al. A systems biology approach investigating the effect of probiotics on the vaginal microbiome and host responses in a double blind, placebo-controlled clinical trial of post-menopausal women. PLoS One. 2014;9:e104511. doi: 10.1371/journal.pone.0104511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Q, Garrity GM, Tiedje JM, Cole JR. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol. 2007;73:5261–5267. doi: 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fernandes AD, Reid JN, Macklaim JM, McMurrough TA, Edgell DR, Gloor GB. Unifying the analysis of high-throughput sequencing datasets: characterizing RNA-seq, 16S rRNA gene sequencing and selective growth experiments by compositional data analysis. Microbiome. 2014;2:15. doi: 10.1186/2049-2618-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Peña AG, Goodrich JK, Gordon JI, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Australian government: national health and medical research council, New Zealand ministry of health. Nutrient reference values for Australia and New Zealandincluding recommended dietary intakes. Canberra, Australia 2006 [Google Scholar]
- 49.Harvie R, Walmsley R, Schultz M. “We are what our bacteria eat”: The role of bacteria in personalizing nutrition therapy in gastrointestinal conditions. J Gastroenterol Hepatol. 2017;32:352–357. doi: 10.1111/jgh.13462. [DOI] [PubMed] [Google Scholar]
- 50.Binder HJ, Mehta P. Short-chain fatty acids stimulate active sodium and chloride absorption in vitro in the rat distal colon. Gastroenterology. 1989;96:989–996. doi: 10.1016/0016-5085(89)91614-4. [DOI] [PubMed] [Google Scholar]
- 51.Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, Yu D, Schilter HC, Rolph MS, Mackay F, Artis D, et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009;461:1282–1286. doi: 10.1038/nature08530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Russo I, Luciani A, De Cicco P, Troncone E, Ciacci C. Butyrate attenuates lipopolysaccharide-induced inflammation in intestinal cells and Crohn’s mucosa through modulation of antioxidant defense machinery. PLoS One. 2012;7:e32841. doi: 10.1371/journal.pone.0032841. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 53.Suzuki T, Yoshida S, Hara H. Physiological concentrations of short-chain fatty acids immediately suppress colonic epithelial permeability. Br J Nutr. 2008;100:297–305. doi: 10.1017/S0007114508888733. [DOI] [PubMed] [Google Scholar]
- 54.Hurst NR, Kendig DM, Murthy KS, Grider JR. The short chain fatty acids, butyrate and propionate, have differential effects on the motility of the guinea pig colon. Neurogastroenterol Motil. 2014;26:1586–1596. doi: 10.1111/nmo.12425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maathuis AJ, van den Heuvel EG, Schoterman MH, Venema K. Galacto-oligosaccharides have prebiotic activity in a dynamic in vitro colon model using a (13)C-labeling technique. J Nutr. 2012;142:1205–1212. doi: 10.3945/jn.111.157420. [DOI] [PubMed] [Google Scholar]
- 56.Chumpitazi BP, Hollister EB, Oezguen N, Tsai CM, McMeans AR, Luna RA, Savidge TC, Versalovic J, Shulman RJ. Gut microbiota influences low fermentable substrate diet efficacy in children with irritable bowel syndrome. Gut Microbes. 2014;5:165–175. doi: 10.4161/gmic.27923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Roager HM, Hansen LB, Bahl MI, Frandsen HL, Carvalho V, Gøbel RJ, Dalgaard MD, Plichta DR, Sparholt MH, Vestergaard H, et al. Colonic transit time is related to bacterial metabolism and mucosal turnover in the gut. Nat Microbiol. 2016;1:16093. doi: 10.1038/nmicrobiol.2016.93. [DOI] [PubMed] [Google Scholar]
- 58.Salonen A, Lahti L, Salojärvi J, Holtrop G, Korpela K, Duncan SH, Date P, Farquharson F, Johnstone AM, Lobley GE, et al. Impact of diet and individual variation on intestinal microbiota composition and fermentation products in obese men. ISME J. 2014;8:2218–2230. doi: 10.1038/ismej.2014.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gorzelak MA, Gill SK, Tasnim N, Ahmadi-Vand Z, Jay M, Gibson DL. Methods for Improving Human Gut Microbiome Data by Reducing Variability through Sample Processing and Storage of Stool. PLoS One. 2015;10:e0134802. doi: 10.1371/journal.pone.0134802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gloor GB, Wu JR, Pawlowsky-Glahn V, Egozcue JJ. It’s all relative: analyzing microbiome data as compositions. Ann Epidemiol. 2016;26:322–329. doi: 10.1016/j.annepidem.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 61.Di Bella JM, Bao Y, Gloor GB, Burton JP, Reid G. High throughput sequencing methods and analysis for microbiome research. J Microbiol Methods. 2013;95:401–414. doi: 10.1016/j.mimet.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 62.Jalanka-Tuovinen J, Salojärvi J, Salonen A, Immonen O, Garsed K, Kelly FM, Zaitoun A, Palva A, Spiller RC, de Vos WM. Faecal microbiota composition and host-microbe cross-talk following gastroenteritis and in postinfectious irritable bowel syndrome. Gut. 2014;63:1737–1745. doi: 10.1136/gutjnl-2013-305994. [DOI] [PubMed] [Google Scholar]
- 63.Kassinen A, Krogius-Kurikka L, Mäkivuokko H, Rinttilä T, Paulin L, Corander J, Malinen E, Apajalahti J, Palva A. The fecal microbiota of irritable bowel syndrome patients differs significantly from that of healthy subjects. Gastroenterology. 2007;133:24–33. doi: 10.1053/j.gastro.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 64.Malinen E, Rinttilä T, Kajander K, Mättö J, Kassinen A, Krogius L, Saarela M, Korpela R, Palva A. Analysis of the fecal microbiota of irritable bowel syndrome patients and healthy controls with real-time PCR. Am J Gastroenterol. 2005;100:373–382. doi: 10.1111/j.1572-0241.2005.40312.x. [DOI] [PubMed] [Google Scholar]
- 65.Lyra A, Rinttilä T, Nikkilä J, Krogius-Kurikka L, Kajander K, Malinen E, Mättö J, Mäkelä L, Palva A. Diarrhoea-predominant irritable bowel syndrome distinguishable by 16S rRNA gene phylotype quantification. World J Gastroenterol. 2009;15:5936–5945. doi: 10.3748/wjg.15.5936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sundin J, Rangel I, Fuentes S, Heikamp-de Jong I, Hultgren-Hörnquist E, de Vos WM, Brummer RJ. Altered faecal and mucosal microbial composition in post-infectious irritable bowel syndrome patients correlates with mucosal lymphocyte phenotypes and psychological distress. Aliment Pharmacol Ther. 2015;41:342–351. doi: 10.1111/apt.13055. [DOI] [PubMed] [Google Scholar]
- 67.Rajilić-Stojanović M, Biagi E, Heilig HG, Kajander K, Kekkonen RA, Tims S, de Vos WM. Global and deep molecular analysis of microbiota signatures in fecal samples from patients with irritable bowel syndrome. Gastroenterology. 2011;141:1792–1801. doi: 10.1053/j.gastro.2011.07.043. [DOI] [PubMed] [Google Scholar]
- 68.Harvie R, Chanyi RM, Burton JP, Schultz M. Using the Human Gastrointestinal Microbiome to Personalize Nutrition Advice: Are Registered Dietitian Nutritionists Ready for the Opportunities and Challenges? J Acad Nutr Diet. 2016 doi: 10.1016/j.jand.2016.10.020. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 69.Ford AC, Moayyedi P. Meta-analysis: factors affecting placebo response rate in the irritable bowel syndrome. Aliment Pharmacol Ther. 2010;32:144–158. doi: 10.1111/j.1365-2036.2010.04328.x. [DOI] [PubMed] [Google Scholar]
- 70.Kelley JM, Lembo AJ, Ablon JS, Villanueva JJ, Conboy LA, Levy R, Marci CD, Kerr CE, Kirsch I, Jacobson EE, et al. Patient and practitioner influences on the placebo effect in irritable bowel syndrome. Psychosom Med. 2009;71:789–797. doi: 10.1097/PSY.0b013e3181acee12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kaptchuk TJ, Shaw J, Kerr CE, Conboy LA, Kelley JM, Csordas TJ, Lembo AJ, Jacobson EE. “Maybe I made up the whole thing”: placebos and patients’ experiences in a randomized controlled trial. Cult Med Psychiatry. 2009;33:382–411. doi: 10.1007/s11013-009-9141-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


