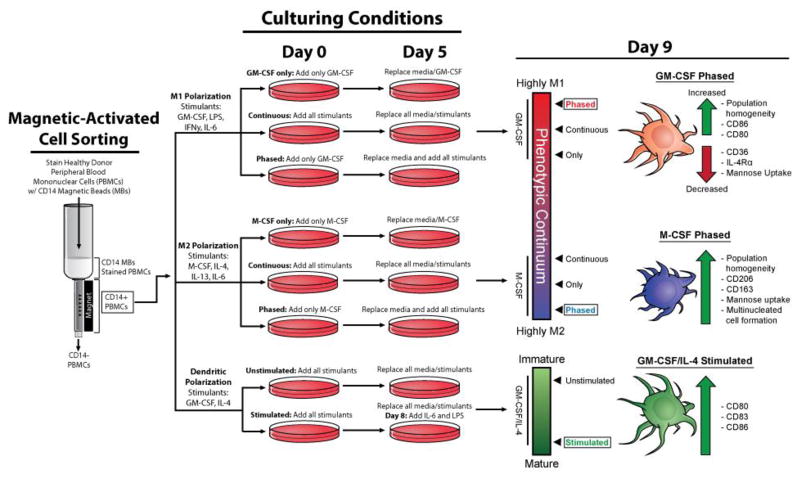
Figure 1. Schematic of Treatment strategies tested to drive monocyte differentiation into homogenous and functional myeloid cell types.
Several polarization strategies were used (GM-CSF only, M-CSF only, continuous or phased). For polarization to the M1 phenotype, CD14+ PBMCs were given GM-CSF (20 ng/ml) for six days and then on day six cells were spiked with GM-CSF in concert with LPS, IL-6, and IFN-γ (20 ng/mL) for an additional four days. For M2 polarization, cells were treated with M-CSF (20 ng/mL) for six days and then on the sixth day, cells were then spiked with M-CSF in concert with IL-4, IL-6 and IL-13 (20 ng/mL) for an additional four days. Isolating human CD14+ monocytes and differentiating them into multiple myeloid cell types for 9 days, we demonstrate that timing, cytokine composition and dosage yields homogenous populations of macrophages and dendritic cells. CD14+ PBMCs under the phased cytokine treatment strategy were driven further than other strategies within the phenotypic continuum to produce homogenous populations that expressed canonical markers of their respective cell types. Under the phased strategy, M2 macrophages also displayed high mannose uptake and cellular fusion. Specific cytokine treatment on day 9 also increased moDC maturation as well as an increase in cell surface markers CD80, CD86, and CD86. We anticipate that the usage of the phased polarization method will be helpful for many immunology research groups as well as cancer researchers seeking to model the tumor microenvironment by incorporation human macrophages.