Fig 1. Biochemical Features of TFII-I.
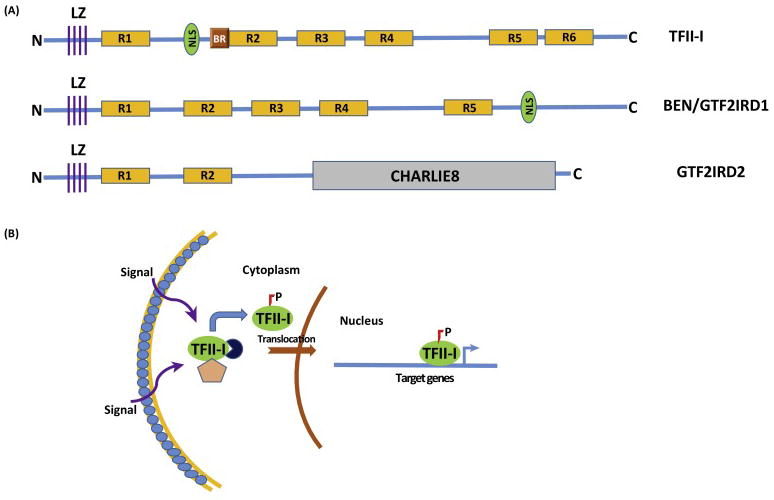
(A) Schematics of 3 TFII-I Family Proteins: The diagram shows TFII-I, GTF2IRD/BEN and GTF2IRD2 proteins. LZ-leucine zipper; R1-R6-TFII-I/GTF2I repeats; NLS-nuclear localization signal; BR-basic region, sequence important for DNA binding; N-N-terminal end and C-Carboxy-terminal end; CHARLIE8-a transposon-like element opf 534 residues (B) Mechanistic Model of Signal-Dependent Nuclear Translocation of TFII-I: Various cell extrinsic signals (such as B and T-cell receptor mediated signals, growth factor receptor stimulation etc) result in phosphorylation of TFII-I and its uncoupling from cytoplasmic tethers (designated by the pink pentagon and navy circle), with subsequent nuclear translocation. Phosphorylated and activated nuclear TFII-I can occupy sites on its target gene promoters (transcription start site is designated by the blue bent arrow) to induce transcription.
